Method for synthesizing propiolic alcohol in simple mode
A kind of propynyl alcohol, a simple technology, applied in the field of preparation of propynyl alcohol, can solve the problems of inconvenient operation, harsh reaction conditions, etc., and achieve the effect of convenient operation, short reaction cycle and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
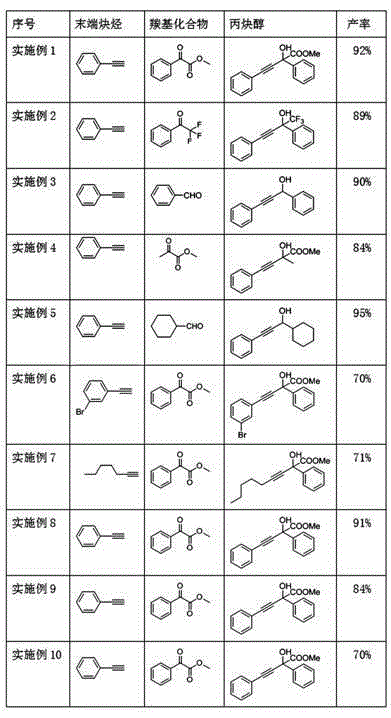
Examples
Embodiment 1
[0026] A kind of method of simply synthesizing propynyl alcohol, be phenylacetylene p-methyl benzoylformate addition reaction in the present embodiment, concrete operation steps are as follows:
[0027] In the atmosphere, add activated zinc powder (3 mmol, 6 equiv), MeI (6 mmol, 12 equiv), phenylacetylene (2 mmol, 4 equiv), 0.5ml N-methylpyrrolidone to a 25ml round bottom flask, room temperature Stir for 2h until the activated zinc powder disappears.
[0028] After the zinc dust disappeared completely, a dichloromethane solution of methyl benzoylformate (0.5 mmol, 1 equiv) was added, and the reaction process was monitored by silica gel thin-layer chromatography, stirred at room temperature for 12 h, the raw material methyl benzoylformate disappeared, and the reaction was stopped. Add 10ml of saturated ammonium chloride aqueous solution and dichloromethane to the reaction solution, stir for 10min, separate the organic phase, extract the aqueous phase with dichloromethane three ...
Embodiment 2
[0032] In this example, the addition reaction of phenylacetylene to α, α, α-trifluoroacetophenone, the specific operation steps are as follows:
[0033] In the atmosphere, add activated zinc powder (3 mmol, 6 equiv), MeI (6 mmol, 12 equiv), phenylacetylene (2 mmol, 4 equiv), 0.5ml N-methylpyrrolidone to a 25ml round bottom flask, room temperature Stir for 2h until the zinc powder disappears.
[0034] After the zinc powder disappeared completely, a dichloromethane solution of α,α,α-trifluoroacetophenone (0.5 mmol, 1 equiv) was added, and the reaction progress was monitored by silica gel thin-layer chromatography, and stirred at room temperature for 12 hours. The raw materials α,α,α- Trifluoroacetophenone disappears, stop reaction, add 10ml saturated ammonium chloride aqueous solution and dichloromethane to reaction liquid, stir 10min, separate organic phase, water phase is extracted three times with dichloromethane, combine organic phase, wash with saturated saline, Dry over a...
Embodiment 3
[0038] Be phenylacetylene to benzaldehyde addition reaction in the present embodiment, concrete operating steps are as follows:
[0039] In the atmosphere, add activated zinc powder (3 mmol, 6 equiv), MeI (6 mmol, 12 equiv), phenylacetylene (2 mmol, 4 equiv), 0.5ml N-methylpyrrolidone into a 25ml round bottom flask, and stir at room temperature 2h, until the activated zinc powder disappears.
[0040] After the zinc powder disappeared completely, add a dichloromethane solution of benzaldehyde (0.5 mmol, 1 equiv), monitor the reaction process by silica gel thin-layer chromatography, stir at room temperature for 12 hours, the raw material benzaldehyde disappeared, stop the reaction, and add 10 ml of saturated chlorine to the reaction solution. Ammonium chloride aqueous solution and dichloromethane were stirred for 10 minutes, the organic phase was separated, the aqueous phase was extracted three times with dichloromethane, the organic phases were combined, washed with saturated b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com