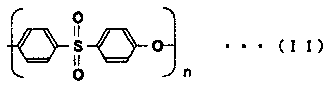Manufacturing method of artificial preservation solution replacing platelet solution
A manufacturing method and technology for platelets, which are applied in chemical instruments and methods, blood filtration, and drug devices, etc., can solve the problems of discovery and hinting without separation methods, and achieve good quality results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
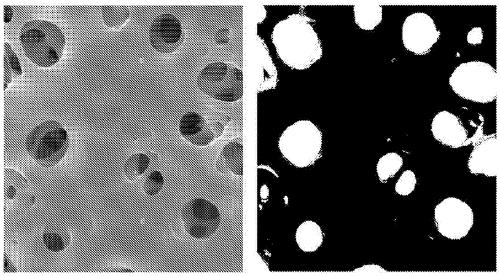
[0169] As the separation membrane, a hollow fiber membrane formed of polysulfone and PVP was used.
[0170]A hollow fiber membrane module (separation membrane module for platelet solution) was produced from the hollow fiber membrane as follows. First, a bundle of 528 hollow fiber membranes obtained by the above-mentioned membrane production operation was inserted into a cylindrical plastic module of φ18×310 mm, immersed in a 60% by mass glycerin aqueous solution, and dried at 50° C. for a whole day and night. Next, 5 mL of a sealing material, which is a polyurethane resin, was injected into both ends of the plastic unit attached to the centrifuge, and rotated at 60 G / 15 minutes (first sealing). After these 15 minutes, 10 mL of sealing material was further injected into both ends of the plastic module, and rotated again at 60 G / 15 minutes (second sealing) to produce a hollow fiber membrane module.
[0171] The inner diameter of the hollow fiber membrane is 300μm, and the membr...
Embodiment 2
[0176] As the separation membrane, a hollow fiber membrane formed of polysulfone and PVP was used.
[0177] A hollow fiber membrane module (separation membrane module for platelet solution) was produced from the hollow fiber membrane as follows. First, a bundle of 50 hollow fiber membranes obtained by the above-mentioned membrane production operation was inserted into a cylindrical plastic module of φ10×220 mm, immersed in a 60% by mass glycerin aqueous solution, and dried at 50° C. overnight. Next, 0.5 mL of a sealing material, which is a polyurethane resin, was injected into both ends of the plastic unit attached to the centrifuge, and rotated at 60 G / 15 minutes (first sealing). After these 15 minutes, 1.7 mL of sealing material was further injected into both ends of the plastic module, and rotated again at 60 G / 15 minutes (second sealing) to produce a hollow fiber membrane module.
[0178] The inner diameter of the hollow fiber membrane is 300μm, and the membrane area is 0...
Embodiment 3
[0183] As the separation membrane, the same hollow fiber membrane formed of polysulfone and PVP as in Example 1 was used. In addition, a hollow fiber membrane module was fabricated in the same manner as in Example 1.
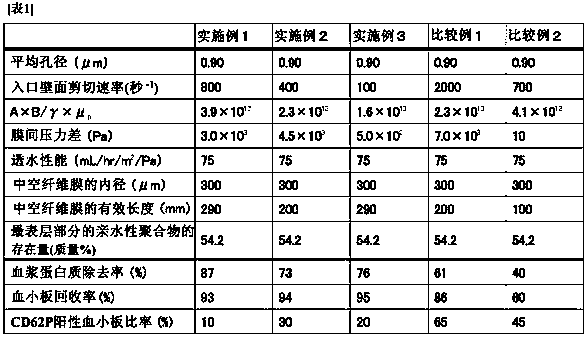
[0184] Using the produced hollow fiber membrane module, the platelet solution was filtered. Specifically, first, 52.2 mL of Meylon manufactured by Otsuka Pharmaceutical Co., Ltd., 126.8 mL of ACD-A solution manufactured by Terumo Corporation, and 3.2 mL of Mg sulfuric acid correction solution (1 mEq / ml) manufactured by Otsuka Pharmaceutical Co., Ltd. were added to 746.2 mL of SOLACET F manufactured by Terumo Corporation. and 71.6 mL of distilled water manufactured by Otsuka Pharmaceutical Co., Ltd. were mixed to prepare M-sol as an artificial preservation solution. In addition, the number of platelets in the original platelet solution is measured in advance.
[0185] For platelets containing 2.0 x 10 11 200 mL of platelet solution (platelet concentration is 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com