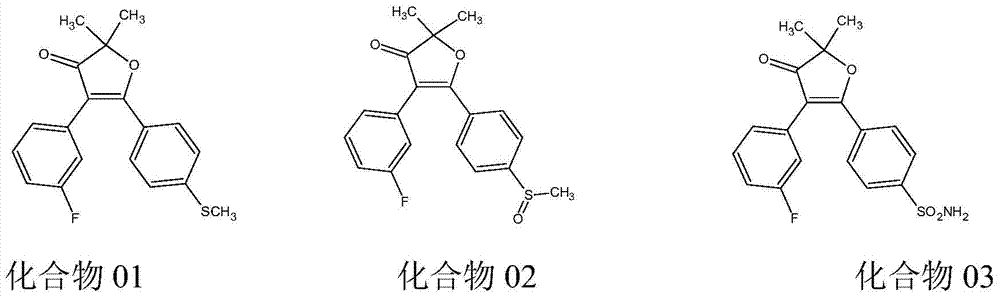
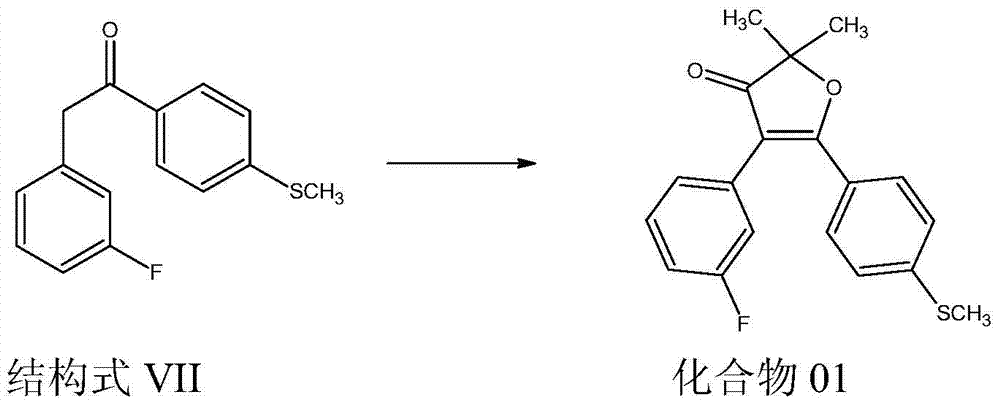
Acyl triazole compounds, acetophenone-substituted phenylmethyl sulfoxide compounds, preparation methods and applications thereof
A technology of phenylmethyl sulfoxide and acyl triazole, which is applied in the preparation of intermediate compounds of pomacoxib and its preparation field, can solve the problems of only 33% yield, high toxicity and poor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Preparation of 1-(2-bromoisobutyryl)-1,2,4-triazole
[0069] Mix 1.38g (20mmol) of 1,2,4-triazole with 13.9ml of tetrahydrofuran, stir, add dropwise 2.29g (10mmol) of pure 2-bromoisobutyryl bromide, drop it in about 30 minutes, and continue stirring for 2 hours. Filter to remove insoluble matter, and the filtrate is concentrated to dryness under reduced pressure. Add 5 ml of methyl tert-butyl ether, stir for 30 minutes, filter, and dry the filter cake to obtain 2.05 g of the product, with a yield of 94%.
[0070] 1 H-NMR (CDCl 3 , 400MHz): δ2.18(S,6H), 8.07(S,1H), 8.97(S,1H)
Embodiment 2
[0071] Example 2: Preparation of 1-(2-chloroisobutyryl)-1,2,4-triazole
[0072] Mix 1.64g (10mmol) of N,N'-carbonylbis(1,2,4-triazole) with 16.4ml of tetrahydrofuran, add 1.23g (10mmol) of 2-chloroisobutyric acid, and stir the reaction at 50°C for 2 hours. Cool and concentrate to dryness under reduced pressure. Add 10 ml of methyl tert-butyl ether, stir for 30 minutes, filter, and dry the filter cake to obtain 1.55 g of the product, with a yield of 89.3%.
[0073] 1 H-NMR (CDCl 3 , 400MHz): δ1.97(S,6H), δ7.93(S,1H), δ8.84(S,1H)
Embodiment 3
[0074] Example 3: Preparation of 1-(2-bromoisobutyryl)-3-methyl-1,2,4-triazole
[0075] Mix 1.66g (20mmol) of 3-methyl-1,2,4-triazole with 16.6ml of tetrahydrofuran, stir, add dropwise 2.29g (10mmol) of pure 2-bromoisobutyryl bromide, and drop it in about 30 minutes. Stirring was continued for 2 hours, filtered to remove insoluble matter, and the filtrate was concentrated to dryness under reduced pressure. Add 5 ml of methyl tert-butyl ether, stir for 30 minutes, filter, and dry the filter cake to obtain 2.09 g of the product, with a yield of 90%.
[0076] 1 H-NMR (CDCl 3 , 400MHz): δ2.17(S,6H), δ2.32(S,3H), δ8.77(S,1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com