Preparation method of progesterone carboxylate
The technology of progesterone and carboxylic acid is applied in the field of preparation of steroid drug intermediates, can solve the problems of low cost, large pollution, long route of carboxylate progesterone, etc., and achieves short synthesis time, high purity yield and abundant resources Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
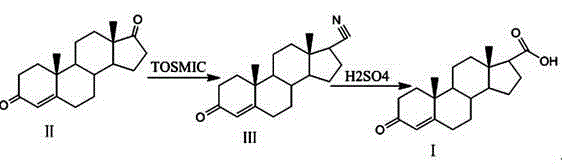
[0018] (1) Cyanide
[0019] Under nitrogen protection, 20g of compound II, namely 4-androstenedione, and 40g of potassium tert-butoxide were added to 800g of ethylene glycol dimethyl ether and 240g of tert-butanol, and cooled to -10°C with stirring; slowly added 30g p-Toluenesulfonylmethyl isocyanide was added, the temperature was raised to 20 °C, stirred for 4 h, TLC until the reaction of the raw materials was complete; the ice water was separated out, and suction filtered to obtain an off-white solid, washed with water until neutral, and dried at 55 °C until At constant weight, 18 g of compound III, 3-oxo-4-ene-17-cyano, were obtained.
[0020] (2) Carboxylation
[0021] 18g of compound III, namely 3-oxo-4-ene-17-cyano, was added to the reaction flask, 144g of 50% sulfuric acid solution was added, the temperature was controlled to 50°C for the reaction, and TLC was monitored until the reaction of the raw materials was complete; Washed with a large amount of water until neu...
Embodiment 2
[0023] (1) Cyanide
[0024] Under nitrogen protection, 20 g of compound II, namely 4-androstenedione, and 60 g of potassium tert-butoxide were added to 900 g of ethylene glycol dimethyl ether and 300 g of tert-butanol, cooled to 0 °C with stirring, and 40 g of tert-butanol was slowly added. Methylbenzenesulfonylmethyl isocyanide, after the addition was completed, the temperature was raised to 25 °C, stirred for 3 h, TLC until the reaction of the raw materials was complete; the ice water was separated out, and the off-white solid was obtained by suction filtration, washed with water until neutral, and dried at 60 °C to constant 18.2 g of compound III, 3-oxo-4-en-17-cyano, were obtained.
[0025] (2) Carboxylation
[0026] 18.2g of compound III, namely 3-oxo-4-ene-17-cyano, was added to the reaction flask, 182g of 50% sulfuric acid solution was added, the temperature was controlled to 60°C for reaction, TLC was monitored until the reaction of the raw materials was complete, 364...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com