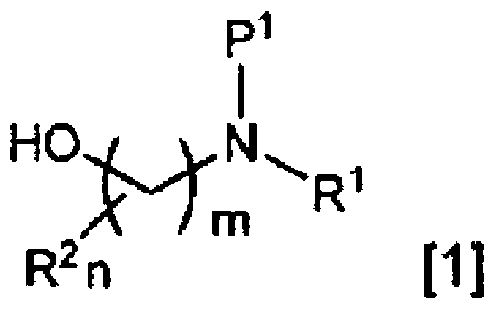
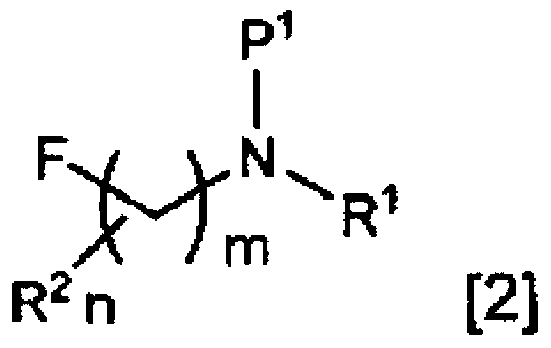
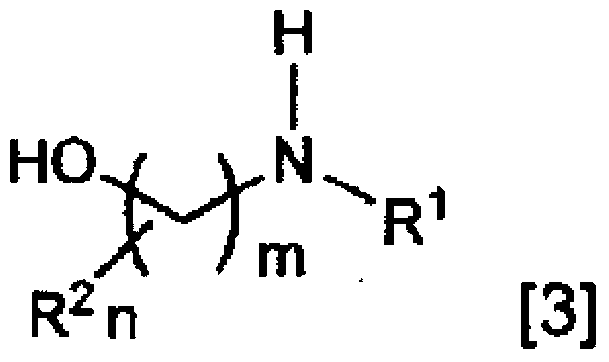
Method for producing fluoroamine
A kind of amine-substituted and fluorinated technology, which is applied in the preparation of sulfonic acid amides, organic chemistry methods, chemical instruments and methods, etc., can solve the problems such as inability to apply secondary amines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Add the following formula to 30.0mL (0.752L / mol) of dichloromethane:
[0138]
[0139] 3.00g (39.9mmol, 1.00eq) of the indicated amino alcohols and 29.0g (287mmol, 7.19eq) of triethylamine were dissolved, and 8.89g (40.1mmol, 1.01eq) of o-nitrobenzenesulfonyl chloride was added at 0°C , and stirred at room temperature for one hour. Add 40.0 mL of water to the reaction-finished solution, extract with 80.0 mL of ethyl acetate, re-extract the recovered aqueous layer with 80.0 mL of ethyl acetate, combine the recovered organic layers, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and dry in vacuo , Purified by column chromatography (silica gel / ethyl acetate:n-hexane=2:1) to obtain the following formula:
[0140]
[0141] The amino alcohol protected body (purified product) shown is 7.93g. The yield is 76%. Gas chromatographic purity was 95.8%. 1 H-NMR is shown below.
[0142] 1 H-NMR (reference substance: Me 4 Si; deuterated solvent: C...
Embodiment 2
[0154] Add the following formula to 2.20L (1.03L / mol) of acetonitrile:
[0155]
[0156] 180g (2.40mol, 1.13eq) of the indicated amino alcohols and 241g (2.38mol, 1.12eq) of triethylamine were dissolved, and 473g (2.13mol, 1.00eq) of o-nitrobenzenesulfonyl chloride was added at 0°C, at room temperature Stir overnight. After completion of the reaction, the precipitated triethylamine hydrochloride was removed by filtration, and the salt was washed with 1.00 L of ethyl acetate. Combine the filtrate and washing liquid, concentrate under reduced pressure to reduce the volume to about 1 / 3, add 1.00L of water, and extract with 1.50L of ethyl acetate. The aqueous layer was re-extracted twice with 500 mL of ethyl acetate, and the combined organic layers were concentrated under reduced pressure. After concentration, add 200mL toluene and then concentrate under reduced pressure to obtain the following formula:
[0157]
[0158] The amino alcohol protected body shown is 533g. Th...
Embodiment 3
[0166] Add the following formula to 53.0 mL (0.987 L / mol) of acetonitrile:
[0167]
[0168] 5.12g (57.4mmol, 1.07eq) of the indicated amino alcohols and 5.66g (55.9mmol, 1.04eq) of triethylamine were dissolved, and 11.9g (53.7mmol, 1.00eq) of o-nitrobenzenesulfonyl chloride was added at 0°C ), and stirred overnight at room temperature. After the reaction was completed, 100 mL of ethyl acetate and 30.0 mL of water were added for double-layer separation, and the aqueous layer was re-extracted twice with 30.0 mL of ethyl acetate. After merging the organic layers, the organic layer was concentrated under reduced pressure after passing through a small amount of silica gel column to obtain the following formula:
[0169]
[0170] 13.2 g of the amino alcohol protected body shown. The yield is 90%. Gas chromatographic purity was 99.0%. 1 H-NMR is described below.
[0171] 1 H-NMR (reference substance: Me 4 Si, deuterated solvent: CDCl 3 ), δppm: 1.82 (m, 2H), 2.65 (br, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com