Synthesis method of (s)-1-(4-diphenyl ether)-2-hydroxy-3-chloropropane
A synthetic method and technology of chloropropane, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of high cost and difficult operation, and achieve the effect of simple operation, low cost, safe and reliable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
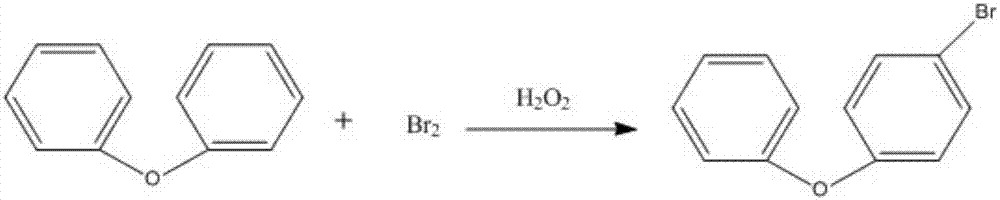
[0036] (1) Put diphenyl ether (44.2g, 0.26mol) into a 250ml four-necked bottle, add (123.5g, (1.25mol) dichloroethane and stir for 1 hour, cool down to -5~0°C, quickly drop into (21g ,0.13mol) bromine, stirred for 1 hour, cooled to -5~0°C, began to drop 30% hydrogen peroxide (15g, (0.13mol), and finished dropping in 0.5~1 hour, the temperature was controlled at 0~5°C After the dropwise addition, the temperature was naturally raised to 25°C, kept at 27°C for 32 hours, quenched with 49g of 15% sodium bisulfite, after phase separation, washed with water until neutral, and distilled to recover dichloro Ethane was distilled under reduced pressure to recover foreboiling diphenyl ether, and finally 51.7 g of 4-bromodiphenyl ether was collected, with a yield of 80.0% and a content of 98.2%.
[0037](2) Under the protection of high-purity nitrogen, 3 g of 4-bromodiphenyl ether, 50 g of tetrahydrofuran, and 3.3 g of magnesium wire were put into a 500 ml four-necked bottle, and the tempe...
Embodiment 2
[0039] (1) Put diphenyl ether (44.2g, 0.26mol) into a 250ml four-necked bottle, add (109g, 1.1mol) dichloroethane and stir for 1 hour, cool down to -5 ~ 0°C, quickly drop into (25.6g, 0.16mol) of bromine, stirred for 1 hour, cooled to -5~0°C, started to add 30% hydrogen peroxide (15g, 0.13mol) dropwise, and finished dropping in 0.5 hour, controlled the temperature between 0~5°C, dropped After the addition, the temperature was naturally raised to 25°C, kept at 28°C for 30 hours, quenched with 49 g of 15% sodium bisulfite, after phase separation, washed with water until neutral, distilled to recover dichloroethane, and reduced Diphenyl ether was recovered by pressure distillation, and 47.8 g of 4-bromodiphenyl ether was finally collected, with a yield of 74.0% and a content of 98.5%.
[0040] (2) Under the protection of high-purity nitrogen, 3 g of 4-bromodiphenyl ether, 71 g of methyl tert-butyl ether, and 3.3 g of magnesium wire were dropped into a 500 ml four-necked bottle, a...
Embodiment 3
[0042] (1) Put diphenyl ether (44.2g, 0.26mol) into a 250ml four-necked bottle, add (136g, 1.37mol) dichloroethane and stir for 1 hour, cool down to -5 ~ 0°C, quickly drop into (17.3g, 0.11mol) of bromine, stirred for 1 hour, cooled to -5~0°C, started to add 30% hydrogen peroxide (15g, 0.13mol) dropwise, and finished dropping in 0.6 hours, controlled the temperature between 0~5°C, dropped After the addition, the temperature was naturally raised to 25°C, kept at 25°C for 48 hours, quenched with 49 g of 15% sodium bisulfite, after phase separation, washed with water until neutral, distilled to recover dichloroethane, and reduced Diphenyl ether was recovered by pressure distillation, and finally 49.3 g of 4-bromodiphenyl ether was collected, with a yield of 76.3% and a content of 98.0%.
[0043] (2) Under the protection of high-purity nitrogen, put 3 g of 4-bromodiphenyl ether, 61 g of ether, and 3.3 g of magnesium wire into a 500 ml four-necked bottle, heat up to 35 ° C and stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com