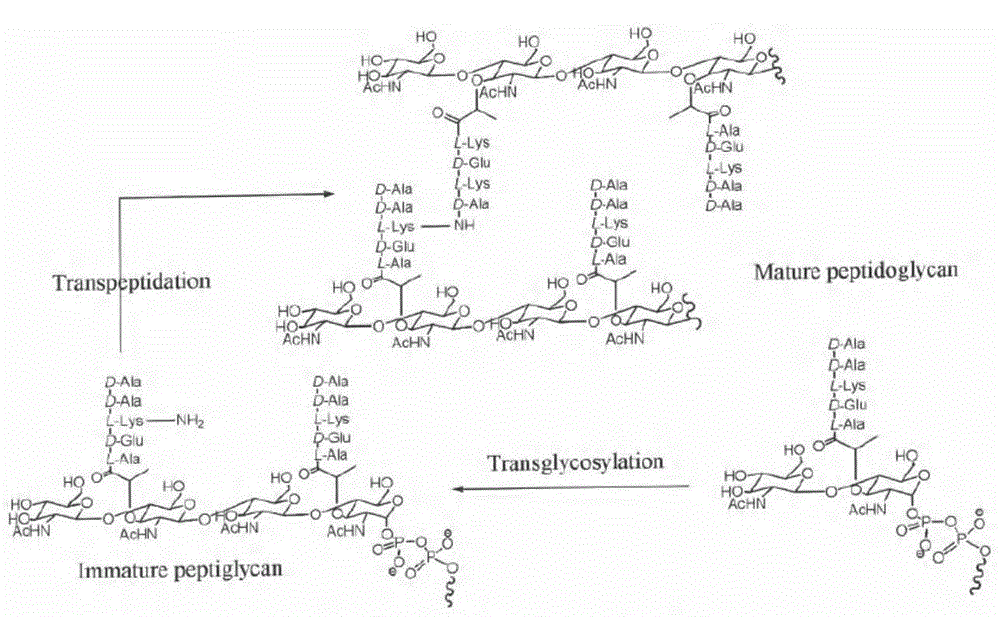
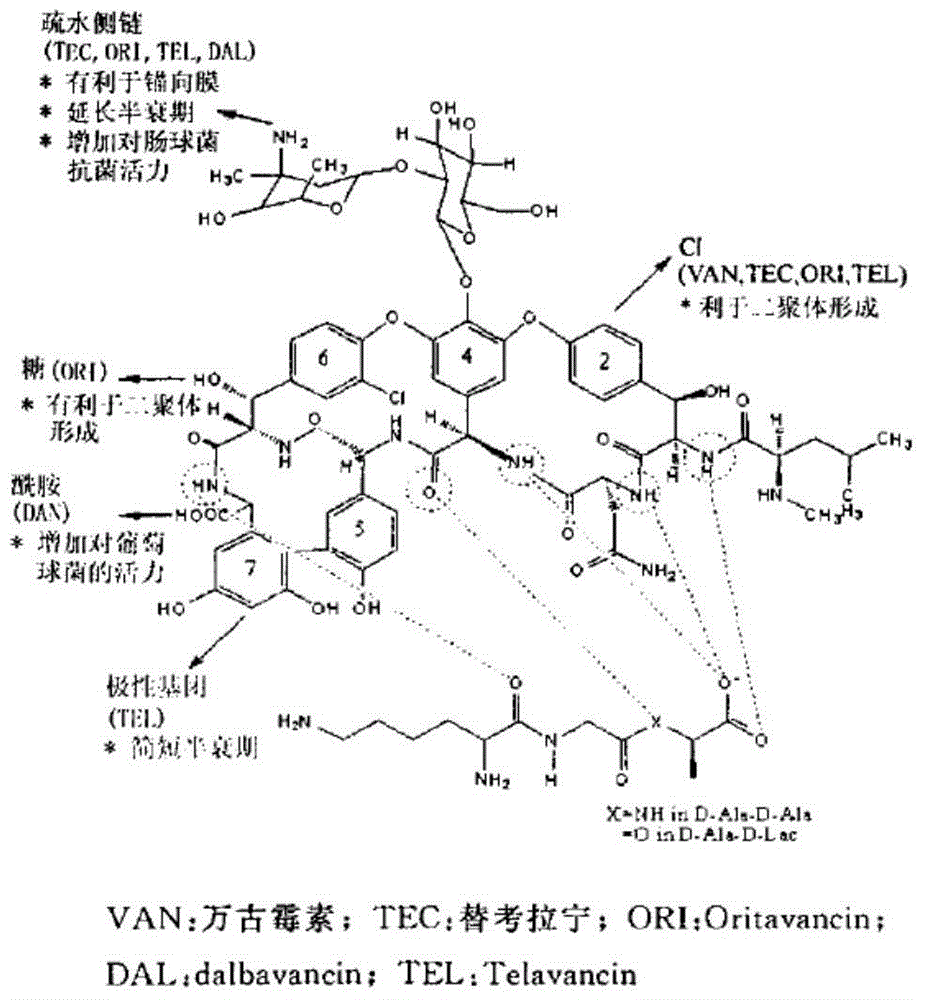
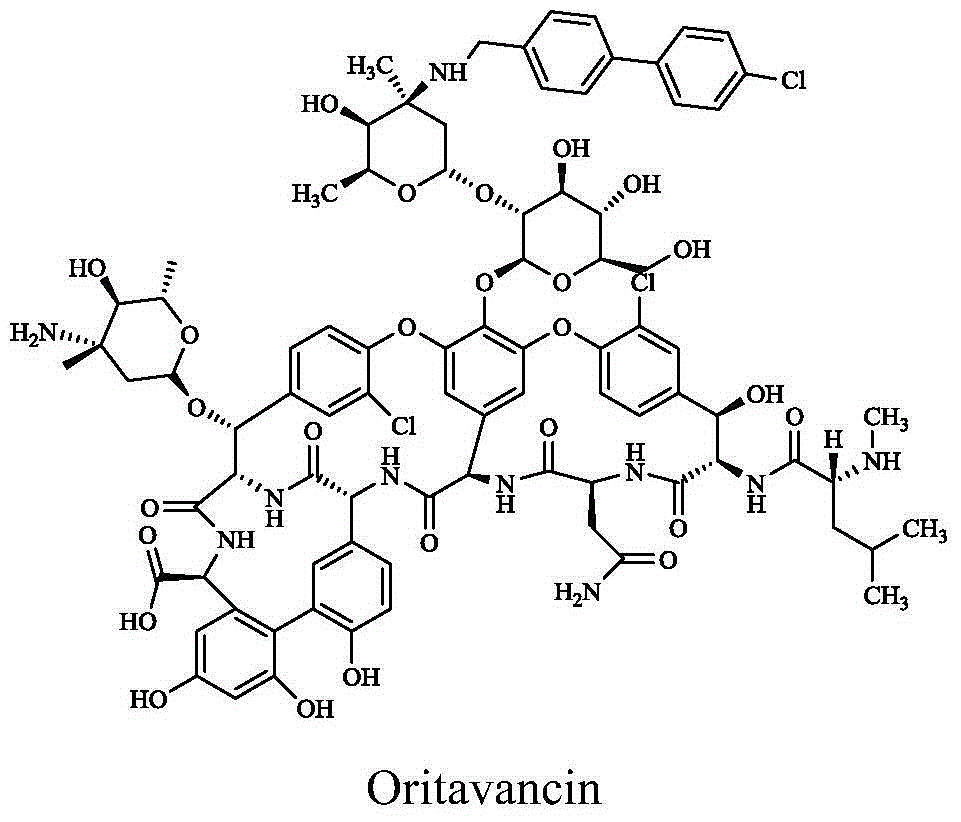
Vancomycin derivative, preparation method and antibacterial application thereof
A technology of drugs and compounds, applied in the field of preparation of vancomycin derivatives, which can solve the problems of increasing membrane permeability and loss of cell viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0132] The synthetic route of preparation example 1 intermediate 2b
[0133]
[0134] Preparation of the first step 3-hexylmercaptopropionic acid 2d
[0135] Add 3-mercaptopropionic acid (2.65g, 25mmol) into the reaction flask, add ethanol (25mL), add 25mL of 2M NaOH solution and stir at room temperature for 5min, then add 1-bromohexane (3.31g, 20mmol) TBAB (0.21g , 0.65mmol) was added, reacted at room temperature for 12h, evaporated the ethanol under reduced pressure, adjusted the pH of the solution to 2~3 with 3M HCl solution, extracted with dichloromethane (25mL×2), combined the organic phases, and washed with saturated saline ( 35mL×2) was washed, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain intermediate 2d, 3.62g of colorless oily liquid, with a yield of 94.71%.
[0136] Preparation of the second step 3-hexylsulfone propionic acid 2e
[0137] Dissolve 3-hexylmercaptopropionic acid 2d (3.59g, 18.88mmol) in 30mL of glacial a...
preparation example 2
[0140] The synthetic route of preparation example 2 intermediate 3b
[0141]
[0142] Preparation of the first step 3-heptylmercaptopropionic acid 3d
[0143] Add 3-mercaptopropionic acid (2.65g, 25mmol) into the reaction flask, add ethanol (25mL), add 25mL of 2M NaOH solution and stir at room temperature for 5min, then add 1-bromo-n-heptane (3.58g, 20mmol) TBAB (0.21 g, 0.65mmol) was added, reacted at room temperature for 12h, evaporated the ethanol under reduced pressure, adjusted the pH of the solution to 2~3 with 3M HCl solution, extracted with dichloromethane (25mL×2), combined the organic phases, and washed with saturated saline (35mL×2), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain intermediate 3d, 3.28g of a colorless oily liquid, with a yield of 80.26%.
[0144] Preparation of the second step 3-heptylsulfone propionic acid 3e
[0145] Dissolve 3-heptylmercaptopropionic acid 3d (3.28g, 16.05mmol) in 25mL of glacial acet...
preparation example 3
[0148] The synthetic route of preparation example 3 intermediate 4b
[0149] Preparation of the first step 3-octylmercaptopropionic acid 4d
[0150] Add 3-mercaptopropionic acid (2.65g, 25mmol) into the reaction flask, add ethanol (25mL), add 25mL of 2M NaOH solution and stir at room temperature for 5min, then add 1-bromo-n-octane (3.86g, 20mmol) TBAB (0.21 g, 0.65mmol) was added, reacted at room temperature for 12h, evaporated the ethanol under reduced pressure, adjusted the pH of the solution to 2~3 with 3M HCl solution, extracted with dichloromethane (25mL×2), combined the organic phases, and washed with saturated saline (35mL×2), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain intermediate 4d, 1.33g of a colorless oily liquid, with a yield of 30.46%.
[0151] Preparation of the second step 3-octylsulfone propionic acid 4e
[0152] Dissolve 3-octylmercaptopropionic acid 4d (1.32g, 6.05mmol) in 25mL of glacial acetic acid, heat and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com