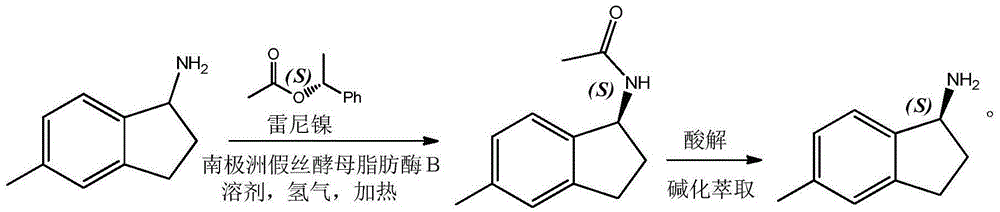
Method for preparing S-5-methyl-1-amino indan
A technology of aminoindan and S-5- is applied in the field of separation and preparation of optically pure chiral compounds to achieve the effects of high optical purity of products, great guidance and application value, and complete utilization of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] 1. Resolution of 5-methyl-1-aminoindan
[0009] In a 1000ML autoclave, add 500ML toluene, 73.6G 5-methyl-1-aminoindan, 90.2g S-1-styryl acetate, 5g Candida plicata lipase and 10g Raney nickel, seal the autoclave, Replace the air in the autoclave with nitrogen, then feed hydrogen into the autoclave to a pressure of 1.5MP, start stirring, and raise the temperature to 45°C for reaction; after 19 hours, take a sample and detect that 5-methyl-1-aminoindane is completely Converted to the acetyl compound of S-5-methyl-1-aminoindane; after the reaction, the solution was concentrated and subjected to column chromatography to obtain pure acetyl compound of S-5-methyl-1-aminoindane 90.2g, the yield is 95.4%.
[0010] 2. Acid hydrolysis to obtain S-5-methyl-1-aminoindan salt
[0011] Take 994.6 g of the acetyl compound of S-5-methyl-1-aminoindane obtained by repeating the previous step several times and add it to the solution mixed with 1000 ml of ethanol and concentrated hydroch...
Embodiment 2
[0015] 1. Resolution of 5-methyl-1-aminoindan
[0016] In a 1000ML autoclave, add 500ML of toluene, 73.6G of 5-methyl-1-aminoindan, 100g of S-1-styroyl acetate, 7g of Candida plicata lipase and 14g of Raney nickel, and seal the autoclave. Replace the air in the autoclave with nitrogen, then feed hydrogen into the autoclave to a pressure of 1.5MP, start stirring, and raise the temperature to 70°C for reaction; after 11 hours, take a sample for detection, 5-methyl-1-aminoindane Completely converted to the acetyl compound of S-5-methyl-1-aminoindane; after the reaction, the solution was concentrated and subjected to column chromatography to obtain the acetyl compound of S-5-methyl-1-aminoindane 89.0 g, the yield is 94.1%.
[0017] 2. Acid hydrolysis to obtain S-5-methyl-1-aminoindan salt
[0018] Take 94.6g of the acetyl compound of S-5-methyl-1-aminoindane obtained by repeating the previous step several times and add it to the solution mixed with 1000ml of ethanol and concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com