A gem-difluoropolycyclic compound and its preparation method
A technology of polycyclic compounds and gem-difluoro compounds, applied in the field of gem-difluoro polycyclic compounds and their preparation, can solve the problems of large restrictions, small quantities, and limited applications, and achieve the effects of increasing acidity, low cost, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
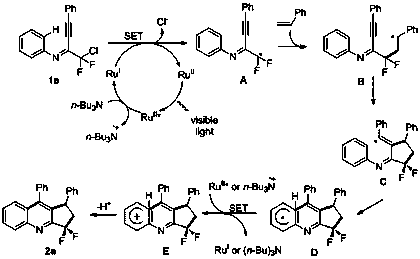
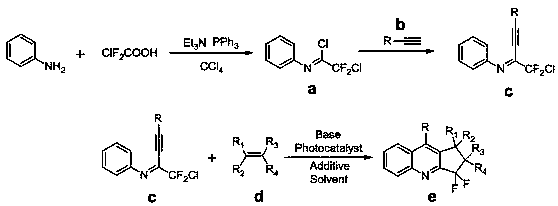
Image
Examples
Embodiment 1
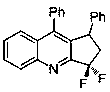
[0046] Example 1 Synthesis of 3,3-difluoro-1,9-diphenylcyclopentaquinoline
[0047] Using phenylacetylene as a reaction alkyne to obtain chlorodifluoromethylalkynimine c, add 1 part of c to a Schlenk bottle, add 2 parts of styrene, 2 parts of potassium carbonate, and 0.01 part of triple Pyridine ruthenium chloride, 0.5 parts of triethylamine and appropriate amount of acetonitrile, after stirring for 12 hours under visible light, the reaction solution is concentrated, and the mixed solvent of petroleum ether / ethyl acetate=20:1 is used as the eluent for column chromatography purification. 3,3-difluoro-1,9-diphenylcyclopentaquinoline was obtained with a yield of 84%, and its structure is shown below:
[0048]
[0049] The NMR data of the compound are as follows:
[0050] 1 H NMR (400 MHz, CDCl3) δ 8.36 (d, J = 8.5 Hz, 1H), 7.75 (ddd, J = 8.4, 6.7, 1.4 Hz, 1H), 7.57 (dd, J = 8.5, 0.9 Hz, 1H) , 7.50 – 7.40 (m, 2H),7.30 (tt, J = 7.5, 1.2 Hz, 1H), 7.27 – 7.23 (m, 1H), 7.08 – 6....
Embodiment 2
[0051] Example 2 Synthesis of 3,3-difluoro-1-(4-methyl)phenyl-9-phenylcyclopentaquinoline
[0052] Using phenylacetylene as a reaction alkyne to obtain chlorodifluoromethylalkynimine c, add 1 part of c to a Schlenk bottle, add 5 parts of 4-methylstyrene and 4 parts of potassium carbonate under a nitrogen atmosphere, 0.01 part of Eosin Y and appropriate amount of acetonitrile, stirred under 1W LED light for 8 hours, then concentrated the reaction solution, and purified by column chromatography with petroleum ether / ethyl acetate=20:1 mixed solvent as eluent to obtain 3,3 -Difluoro-1-(4-methyl)phenyl-9-phenylcyclopentaquinoline, the yield is 87%, and its structure is shown below:
[0053]
[0054] The NMR data of the compound are as follows:
[0055] 1 H NMR (400 MHz, CDCl 3 ) δ 8.39 (d, J = 8.5 Hz, 1H), 7.78 (ddd, J =8.4, 6.7, 1.4 Hz, 1H), 7.60 (dd, J = 8.5, 0.9 Hz, 1H), 7.53 – 7.43 (m,2H),7.34 (tt, J = 7.5, 1.2 Hz, 1H), 7.30 – 7.25 (m, 1H), 7.10 (td, J = 7.6, 0....
Embodiment 3
[0056] Example 3 Synthesis of 3,3-difluoro-1-(4-methoxy)phenyl-9-phenylcyclopentaquinoline
[0057] Use phenylacetylene as the reaction alkyne to obtain chlorodifluoromethylalkynimine c, add 1 part of c to the Schlenk bottle, add 3 parts of 4-methoxystyrene and 3 parts of sodium carbonate under nitrogen atmosphere , 0.005 parts of Rose Bengal and 0.005 parts of triethylamine and acetonitrile solvent, after stirring for 4 hours under 1W LED light, the reaction solution was concentrated, petroleum ether / ethyl acetate=20:1 mixed solvent as eluent column layer Analysis and purification can obtain 3,3-difluoro-1-(4-methoxy)phenyl-9-phenylcyclopentaquinoline with a yield of 87%, and its structure is as follows:
[0058]
[0059] The NMR data of the compound are as follows:
[0060] 1 H NMR (400 MHz, CDCl 3 ) δ 8.36 (d, J = 8.5 Hz, 1H), 7.76 (ddd, J =8.4, 6.7, 1.5 Hz, 1H), 7.56 (dd, J = 8.4, 0.9 Hz, 1H), 7.51 – 7.45 (m, 1H),7.38 (t, J = 7.5 Hz, 1H), 7.29 – 7.24 (m, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com