Dual-reagent glycosylated hemoglobin detection kit
A technology of glycosylated hemoglobin and detection kits, applied in the biological field, can solve problems such as reagent instability, manual operation errors, and differences in test results, and achieve the effects of good reagent stability, control of batch-to-batch differences, and cost savings for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
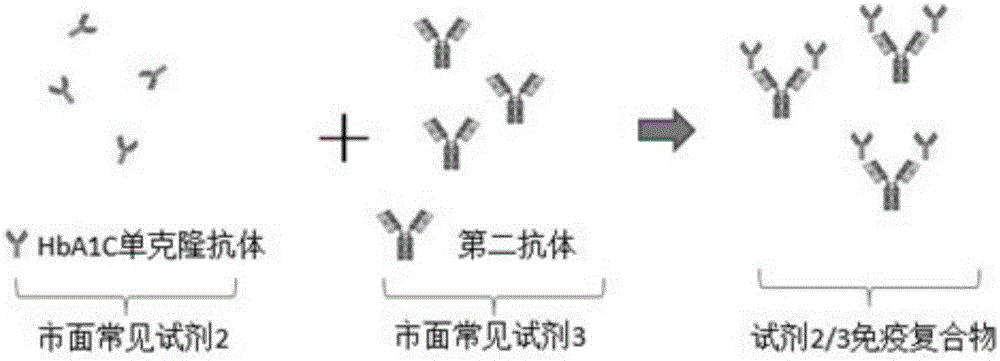
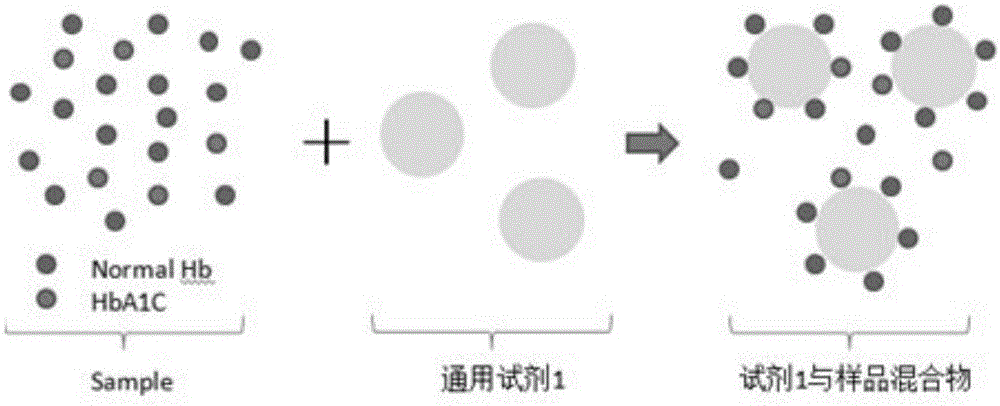
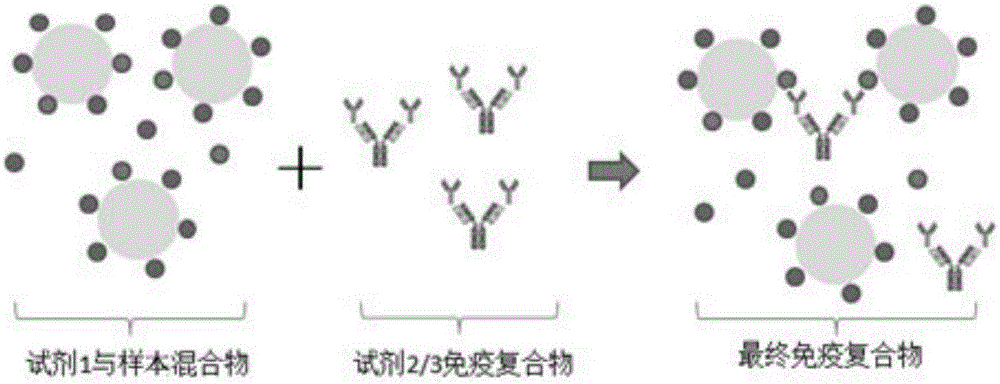
[0044] A two-reagent glycosylated hemoglobin detection kit, including reagents: a latex microsphere solution (reagent 2) labeled with a monoclonal antibody that specifically recognizes glycosylated hemoglobin replaces the traditional detection reagent HbA1C monoclonal antibody (traditional reagent 2) and the corresponding secondary antibody ( Traditional reagent 3), usually the HbA1C monoclonal antibody is a mouse monoclonal antibody, and the secondary antibody is an anti-mouse IgG antibody. This reagent 2 uses a chemical coupling method to label a monoclonal antibody that specifically recognizes glycosylated hemoglobin, referred to as HbA1C monoclonal antibody, on prepared on latex microspheres. Wherein, the particle size of the latex microsphere particles is 100 nm.
[0045] In this embodiment, direct quantitative detection of the content percentage of glycated hemoglobin in the test sample is carried out by immunoturbidimetric method. The experimental process is as follows...
Embodiment 2
[0052] A two-reagent glycated hemoglobin detection kit, according to the detection method of Example 1, three batches of Muti-Mab (polyglycosylated hemoglobin monoclonal antibody), as shown in Table 1, and three batches of the kit of the present invention , as shown in Table 2, also using the common blank latex solution (reagent 1) in the market, carried out the calibration test according to the general parameters, and compared the difference between batches of absorbance data, the results are as follows Figure 10 , Figure 11 As shown, the difference in the absorbance values of the three batches of Muti-Mab is large, and the relative deviation of the absorbance values is greater than 15%; while the difference between batches of the kit of the present invention is small, which meets the requirement that the difference between batches of conventional in vitro diagnostic reagents is less than 15%.
[0053] Table 1
[0054]
[0055] Table 2
[0056]
Embodiment 3
[0058] A double-reagent glycated hemoglobin detection kit, the preferred 50-200nm latex microspheres according to the detection method of embodiment 1 are used as HbA1C monoclonal antibody labeling, using 50nm ( Figure 12 ), 100nm ( Figure 13 ), 150nm ( Figure 14 ), 200nm ( Figure 15 ) latex microspheres were coupled with the same amount of commercial HbA1C monoclonal antibody according to the conventional chemical coupling method, and the clinical results were compared with those of common kits on the market such as Point reagent (traditional reagent 2 and traditional reagent 3 need to be mixed in advance), the accuracy of the results All are ideal, the correlation R of clinical results is greater than 0.98, and the relative deviation is less than 10%. When latex microspheres with smaller particle size are used, the antibody labeling process is prone to agglomeration, which makes it difficult to control the difference between batches of labeling. For example, if 30nm la...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com