Method and system for reevaluating safety of drug after appearance on market
A safety and drug technology, applied in the direction of instruments, data processing applications, prediction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Re-evaluation of the safety of salvianolate for injection after marketing
[0047] The specific methods and steps include:
[0048] (1) Determine the research drug: determine that the research drug of this project is salvianolate for injection, and conduct research on the relevant information of the instructions, its indications, usage and dosage, contraindications, adverse reactions, etc. The indications are: Promoting blood circulation, removing blood stasis, dredging the veins. It is used for stable angina pectoris of coronary heart disease, graded as grade Ⅰ or Ⅱ. The symptoms of angina pectoris are mild to moderate, and the TCM syndrome is heart-blood stagnation syndrome. Symptoms include chest pain, chest tightness, and palpitations.
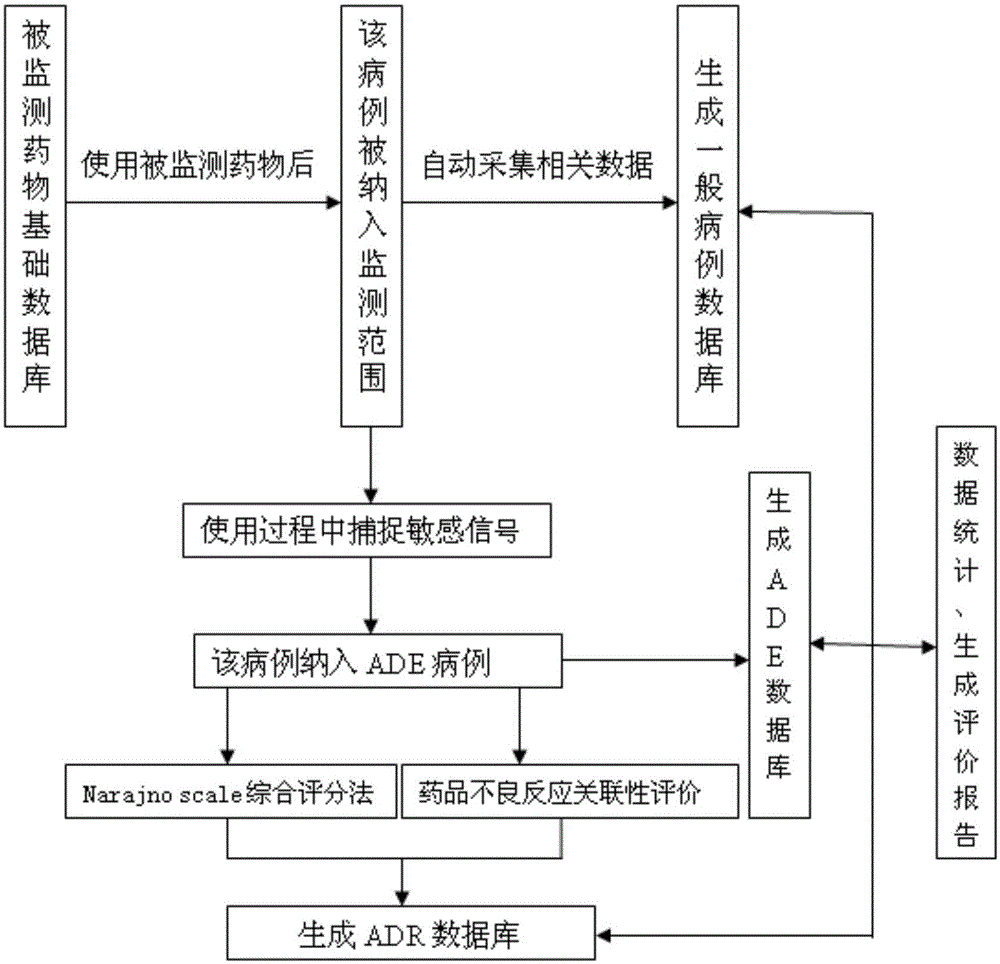
[0049] (2) Set sensitive signal: According to the purpose of the study is to understand the influencing factors of clinical suspected anaphylaxis of salvianolate for injection, this study only needs to set a sensitive si...
Embodiment 2
[0057] Example 2: Using the drug safety re-evaluation system to conduct post-marketing safety re-evaluation of Shenmai Injection
[0058] The specific content and operation steps are as follows:
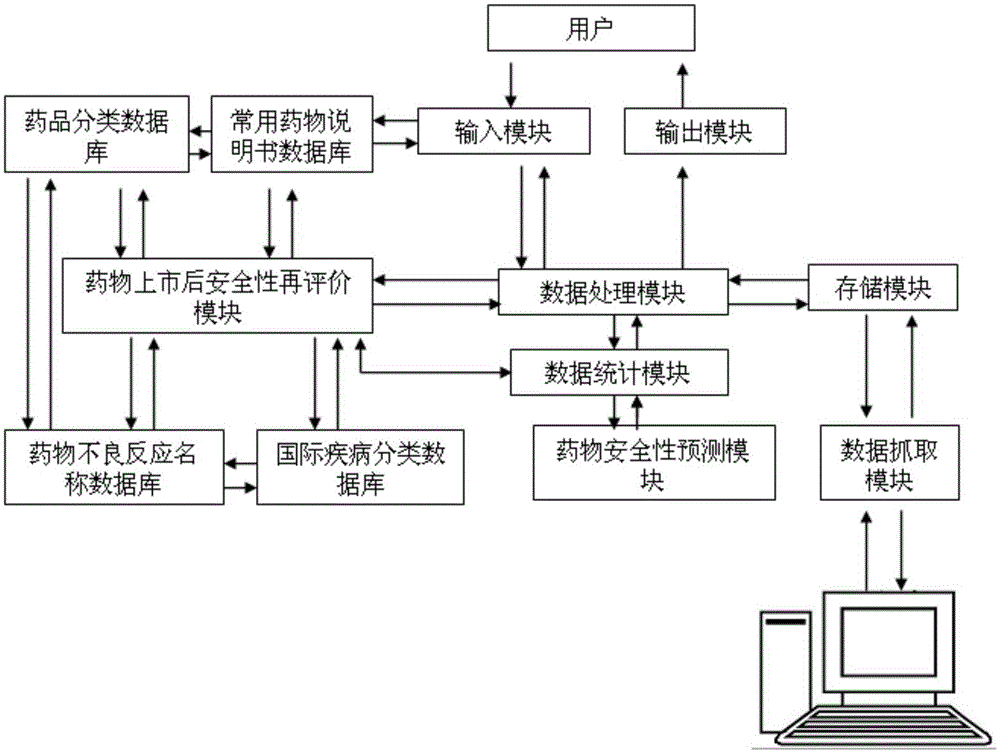
[0059] (1) Determine the research drug, set the sensitive signal, and determine the number of cases collected: the setting of the research drug and its sensitive signal is realized through the input module, and the execution steps are as follows:
[0060] ①Create a new Shenmai injection research project and create a directory of research documents;
[0061] ②Shenmai Injection is displayed as a new research project in the system, and the research drug information is saved. Enter the indications, contraindications, usage and dosage of the study, its sensitive signals, and the number of cases collected according to the instructions.
[0062] The relevant information set by Shenmai injection according to the instruction manual includes: indications, usage and dosage, contraindications, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com