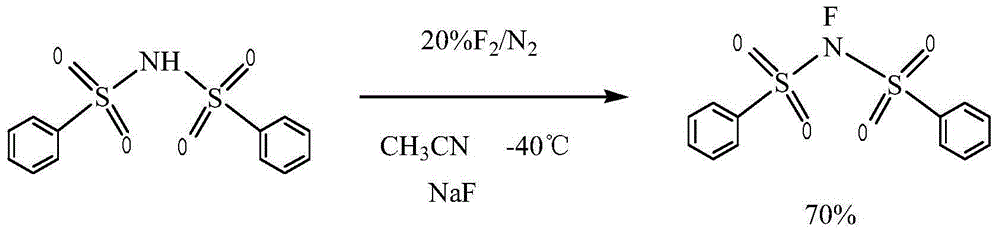
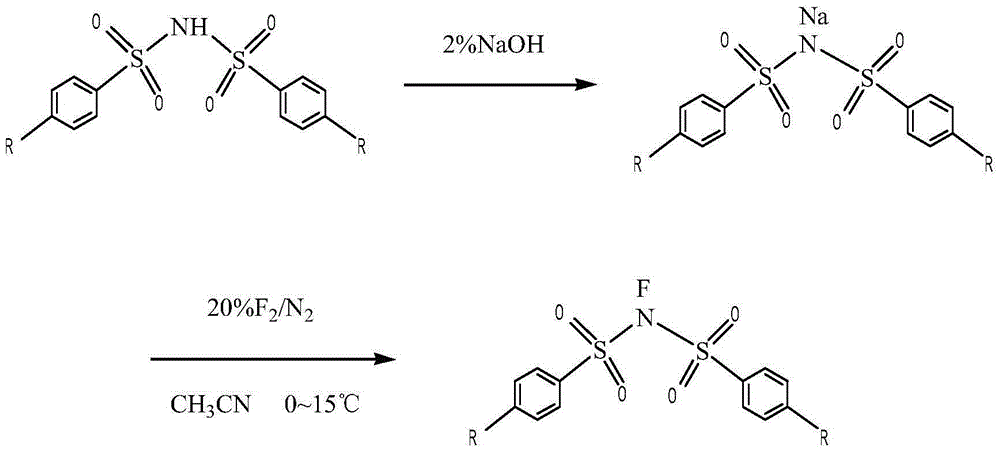
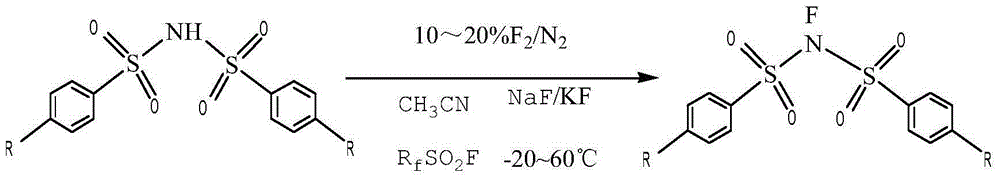Novel process for preparing N-fluorobenzenesulfonimide with one-step method
A fluorobisbenzenesulfonimide, a new process technology, applied in the field of one-step preparation of N-fluorobisbenzenesulfonimide, can solve the problem of low reaction temperature, low yield, N-fluorobisphenyl Solve the problem of unsafe preparation reaction of sulfonimide, and achieve the effect of less side reactions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
[0027] The synthesis of embodiment 1-15N-fluorinated bisbenzenesulfonylimide
[0028] First, fully stir the p-substituted bisbenzenesulfonimide, metal fluoride and perfluoroalkylsulfonyl fluoride with industrial acetonitrile; The gas is introduced from the final reaction tower; during the reaction process, the flow rate of the fluorine-nitrogen mixed gas is controlled to ensure that the reaction is carried out gently between -20 ° C and 60 ° C; after the reaction is completed, suction filtration, washing, and recrystallization are obtained. N-fluorobisbenzenesulfonimide.
[0029] Table 1 lists the data for the synthesis of N-fluorobisbenzenesulfonimide under different reaction conditions.
[0030] The list of experimental data of table 1 embodiment
[0031]
[0032]
[0033]
[0034] The above data show that:
[0035] 1: Whether to add perfluorobutylsulfonyl fluoride to the reaction system will significantly affect the yield of N-fluorobisbenzenesulfonimide. The o...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap