Nanostrip dopo-hq cyclotriphosphazene derivative and preparation method thereof
A DOPO-HQ, cyclotriphosphazene technology, applied in the field of DOPO-HQ cyclotriphosphazene derivatives and their preparation, achieves the effects of no inflammatory reaction, good biocompatibility, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
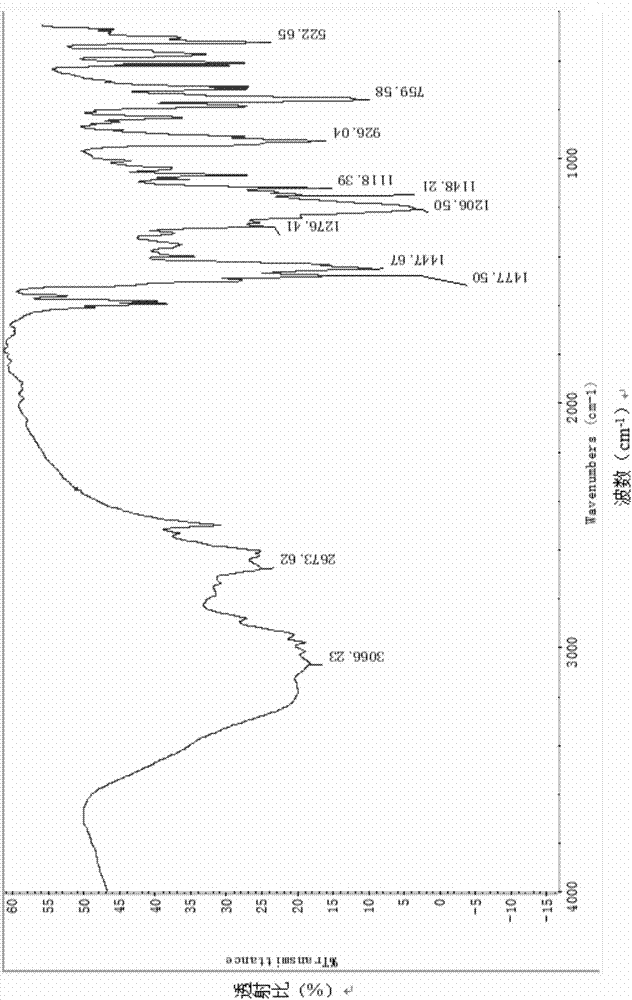
[0022] Specific embodiment one: the preparation method of the nanostrip DOPO-HQ cyclotriphosphazene derivative in this embodiment is carried out according to the following steps:
[0023] In the atmospheric distillation device, add about 50mL of triethylamine, and collect the distillate at 89-90°C for later use.
[0024] Add 1.36g of DOPO-HQ and 80mL of tetrahydrofuran into a 250mL three-necked flask equipped with an ultrasonic mixing device, and ultrasonically dissolve. Measure 2.0 mL of refined triethylamine, add DOPO-HQ in tetrahydrofuran solution, and mix for 5 minutes. Weigh 0.5 g of hexachlorocyclotriphosphazene, add 30 mL of tetrahydrofuran and stir to dissolve, then slowly drop it into the reactor through a constant pressure dropping funnel. After the dropwise addition, continue to react for 5 hours, and a large amount of white solids precipitate out. Most of the tetrahydrofuran solvent was removed by rotary evaporation, the concentrated solution was poured into a 50m...
specific Embodiment approach 2
[0025] Specific embodiment two: the preparation method of the nanostrip DOPO-HQ cyclotriphosphazene derivative in this embodiment is carried out according to the following steps:
[0026] Step 1. Add 4.2mmol dry DOPO-HQ to 100mL tetrahydrofuran, stir until completely dissolved;
[0027] Step 2, then add 0.5mL refined triethylamine (collected 89~90°C distillate after Tianjin Kemiou AR distillation), add dropwise (drop speed 1mL / min) tetrahydrofuran solution of hexachlorocyclotriphosphazene (1mmol hexachloro Cyclotriphosphazene was prepared by adding 20mL of tetrahydrofuran), and then ultrasonically refluxed for 10h under the conditions of ultrasonic frequency of 40HZ, power of 210W, and reaction temperature of 70°C;
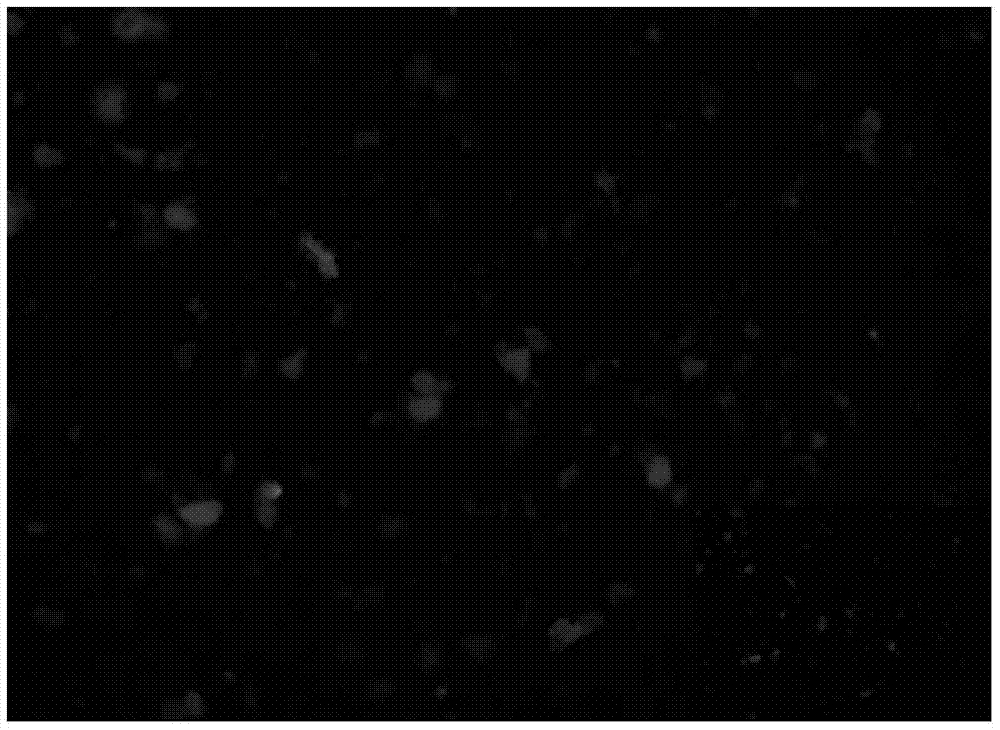
[0028] Step 3, then centrifuge (6000r / min, 20min), wash with 30mL acetone and distilled water ultrasonically (210W, 2min), respectively, to obtain (fluorescent nanostrips) DOPO-HQ cyclotriphosphazene derivative;
[0029] The DOPO-HQ cyclotriphosphazene derivative...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com