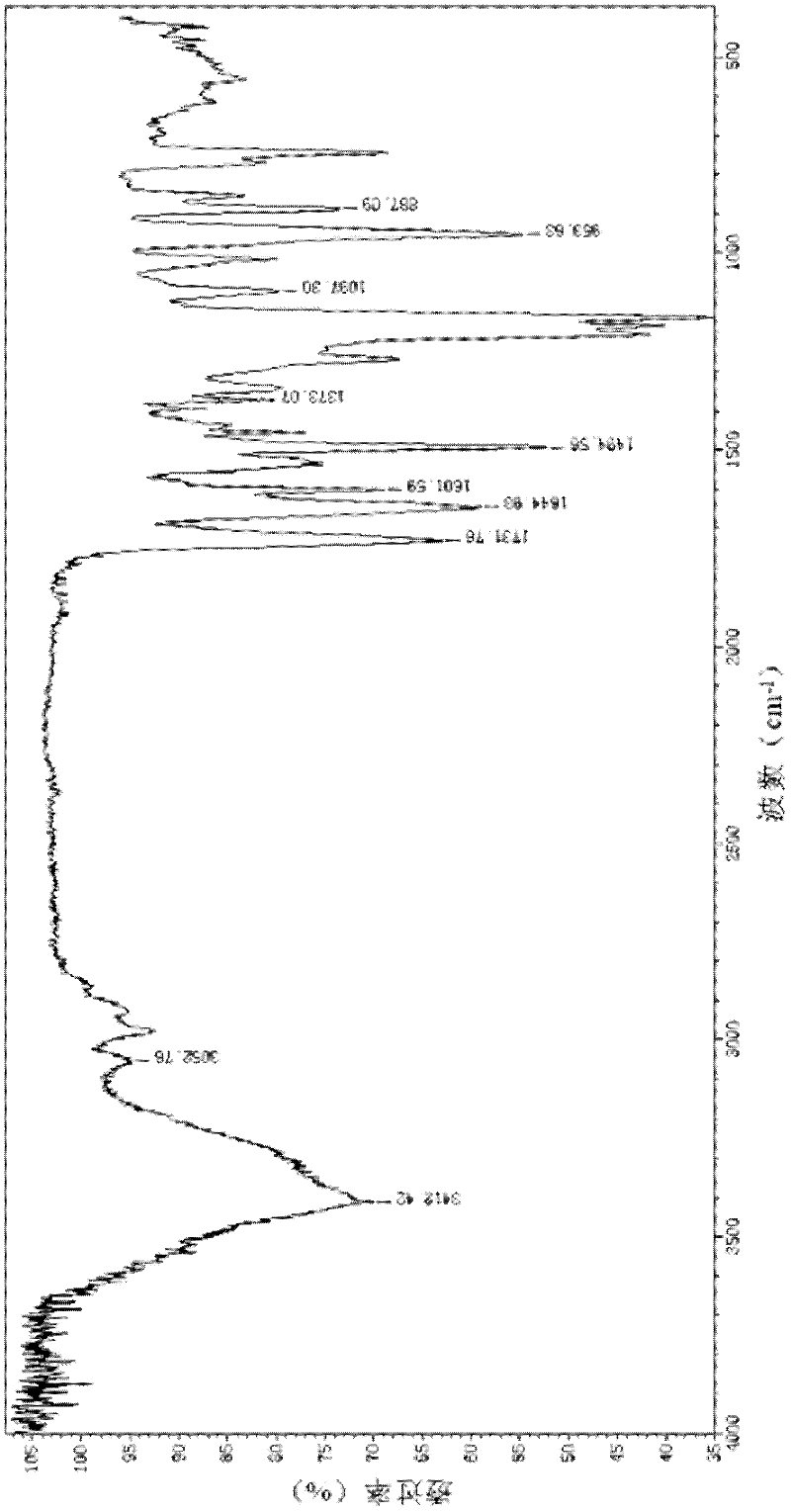
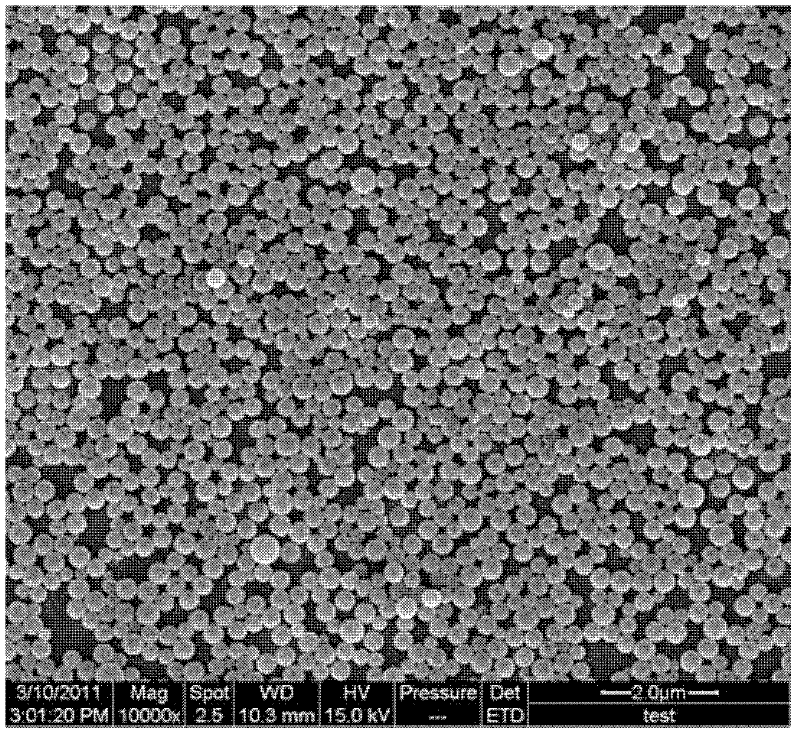
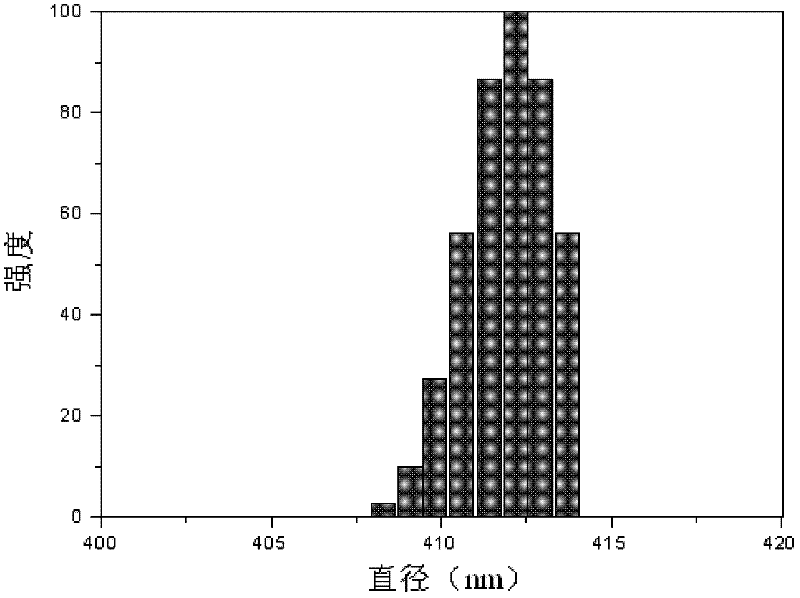
Hexaminoacid ester phenoxyl cyclotriphosphazene, its fluorescent nano-microsphere and preparation method thereof
A technology of ester phenoxy cyclotriphosphazene and carboxyphenoxy cyclotriphosphazene is applied in the field of cyclotriphosphazene and its fluorescent nano-microspheres and preparation, and achieves the effects of simple process, high yield and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0024] Specific embodiment one: the present embodiment is six amino acid ester phenoxycyclotriphosphazene, and its general structural formula is as follows:
[0025]
[0026] Wherein, M is an amino acid ester, and the amino acid in the amino acid ester is glycine, tryptophan, phenylalanine, alanine, valine, leucine, isoleucine or methionine; Certain esters are methyl, ethyl or benzyl.
[0027] The hexaamino acid ester phenoxycyclotriphosphazene of this embodiment is a light yellow powder with a stable structure, good biocompatibility, easy degradation, and the advantages of non-toxic degradation products and no inflammatory reaction. The ester bond in the hexaamino acid ester phenoxycyclotriphosphazene of the present invention is easily hydrolyzed in the acidic environment of the human body, and the amino acid obtained further promotes the degradation of the whole molecule in the body, and the degradation products are phosphoric acid, ammonia and alcohol, which are non-toxi...
specific Embodiment approach 2
[0028] Embodiment 2: This embodiment is different from Embodiment 1 in that M is glycine methyl ester or tryptophan ethyl ester. Other parameters are the same as in the first embodiment.
specific Embodiment approach 3
[0029]Specific embodiment three: This embodiment is the preparation method of hexaamino acid ester phenoxycyclotriphosphazene as described in specific embodiment one, which is achieved through the following steps: 1. The dried six-p-carboxyphenoxy Cyclotriphosphazene is put into the four-necked flask, and then the reaction device is obtained after installation of nitrogen drying, spherical condenser, thermometer, stirring, gas recovery and heating device, and then nitrogen is blown into the reaction device; 2. In the four-necked flask Add thionyl chloride, then reflux and stir at 60-90°C for 2-20 hours, and then separate the remaining thionyl chloride from the reaction device to obtain hexa-p-formyl chloridephenoxycyclotriphosphazene; Add tetrahydrofuran into the bottle, stir to dissolve, then add amino acid ester hydrochloride and acid-binding agent, and then reflux at 60-90°C for 20-36 hours to obtain the post-reaction system, then concentrate the post-reaction system, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com