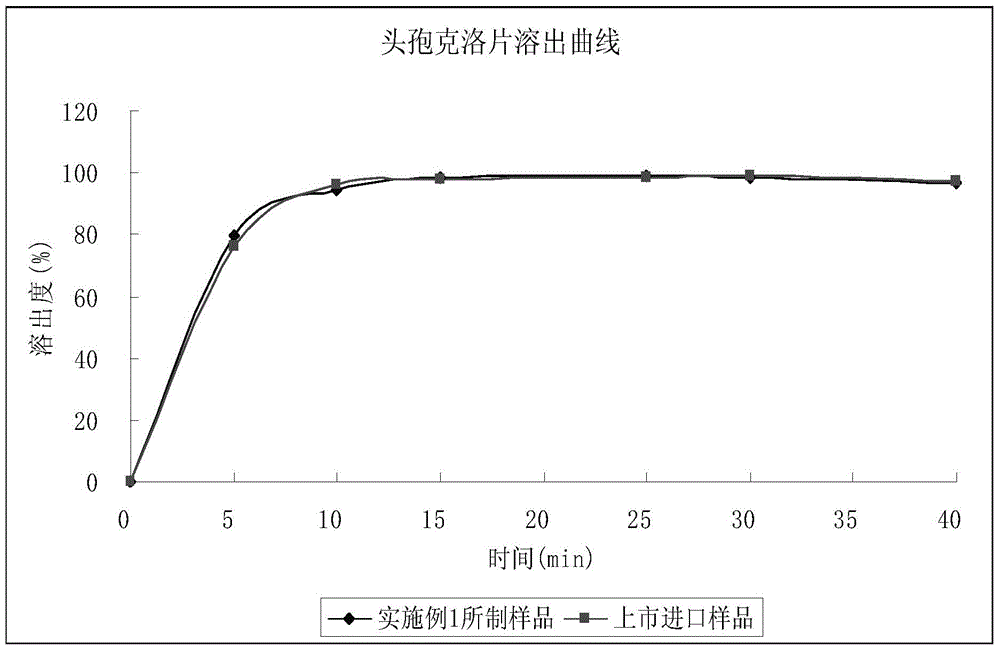
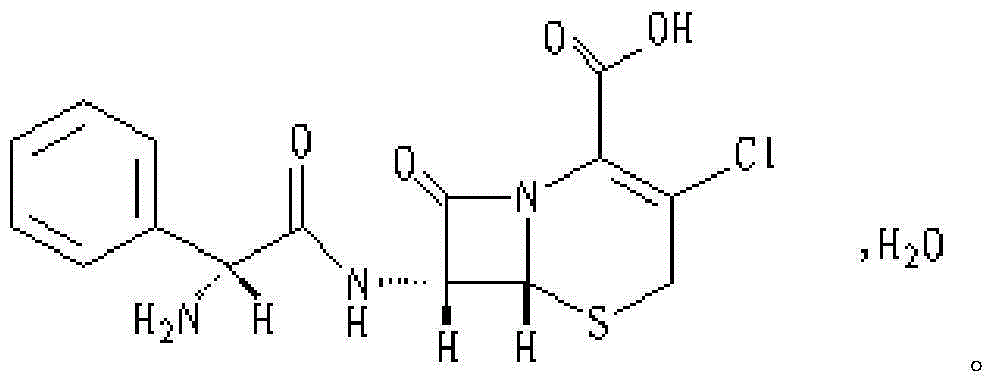
Cefaclor tablet composition as well as preparation method and application thereof
A technology of cefaclor tablet and cefaclor, applied in the field of pharmaceutical composition of cefaclor tablet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 The present invention adopts dry granulation to prepare the technique of cefaclor tablet
[0032] (1) Preparation process: take cefaclor raw material and pass through 80-mesh sieve, and pass through 80-mesh sieve for auxiliary materials except flow aid respectively, and mix evenly in a CH50V mixer with a rotating speed of 25-30 rpm and a mixing time of about 30 Minutes, the mixed material is granulated with GL-5B dry granulator, feeding speed: 55-65rpm, 250-300g / min, pressing wheel speed: 5-7rpm, pressing wheel spacing: 0.1-0.3mm, granulation speed: 298rpm, the qualified granules are controlled at 20-40 meshes, the angle of repose of the granules is measured to be less than 30°, the flow aid of the prescribed amount is added, placed in a ZP35D tablet press for tableting, the speed of the tablet press is adjusted to 13 rpm, and the tablet pressure is controlled to 7~10kg. Film coat Opadry, the film coating premix, with purified water.
[0033] (2) The fo...
Embodiment 2
[0037] (1) preparation process is with embodiment 1;
[0038] (2) The formula of cefaclor tablet composition is shown in Table 2:
[0039] Table 2, cefaclor tablet formula 2
[0040] component name
Embodiment 3
[0042] (1) preparation process is with embodiment 1;
[0043] (2) The formula of cefaclor tablet composition is shown in Table 3:
[0044] Table 3, cefaclor tablet formula 3
[0045] component name
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com