Micelle polypeptide vaccine with poly(ethylene glycol)-phosphatidylethanolamine as carrier
A pegylated phospholipid and polypeptide vaccine technology, which is applied in the field of medicine and biology, can solve the problems of few studies on protein or polypeptide entrapment and no reports, and achieve the effect of improving the cure rate and survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1, the preparation of micelle E7 vaccine
[0066] The E7 antigen polypeptide is a tumor-associated antigen derived from human papillomavirus type 16 (HPV16), and a palmitic acid molecule is connected to the N-terminal, that is, the E7-20 polypeptide; E7 43-62 Sequence: Palmitate-GQAEPDRAHYNIVTFCCKCD;
[0067] Stock solution preparation:
[0068] (1) Add 1mL of anhydrous methanol to each tube of 1mg E7-20 that has been dispensed to make a stock solution of E7-20 with a concentration of 1mg / mL;
[0069] (2) Dissolving the MPLA powder in a 1:2 mixed solution of methanol and chloroform to prepare a MPLA stock solution with a concentration of 1 mg / mL;
[0070] (3) Weigh 30 mg of PEG-PE powder and add 3 mL of chloroform to dissolve to prepare 10 mg / mL PEG-PE stock solution.
[0071] 1. Preparation of micellar vaccine solution, prepare 10mL micellar vaccine according to the prescription in Table 1:
[0072] Step (1) Take 5mL of PEG-PE stock solution in a rotary ...
Embodiment 2
[0081] Example 2, Determination of Encapsulation Efficiency of Fatty Acidated E7 / OT-1 Antigen Micelles
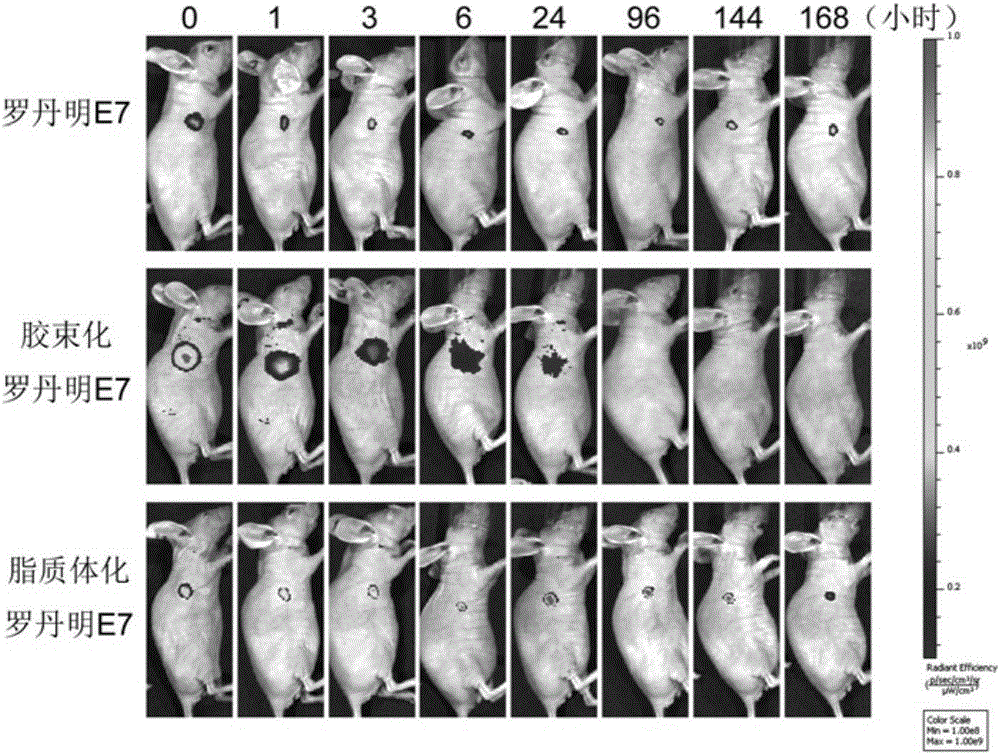
[0082] Synthesize fatty acidated E7 antigenic polypeptide and OT-1 antigenic polypeptide (palmitic acid-EQLESIINFEKLTEWKD) with rhodamine fluorescence modification, the free fattyated antigenic polypeptide cannot dissolve in water and thus form a suspension, and the fattyated antigenic polypeptide is PEG -After the PE micelles are loaded, a stable solution is formed, so that the free antigenic polypeptide and the antigenic polypeptide loaded by the micelles can be separated, and then calculated according to the change of the content of the antigenic polypeptide loaded by the micelles in the solution Encapsulation rate of antigen polypeptide, encapsulation rate = packaged antigen amount / total antigen amount x100%.
[0083] According to the ratios in Table 2 and Table 3, micelles containing different proportions of polypeptides were prepared, and the preparation process was t...
Embodiment 3
[0088] Embodiment 3, the physical properties characterization of micellar polypeptide vaccine
[0089] 1. Morphological observation of micellar E7 vaccine by transmission electron microscope
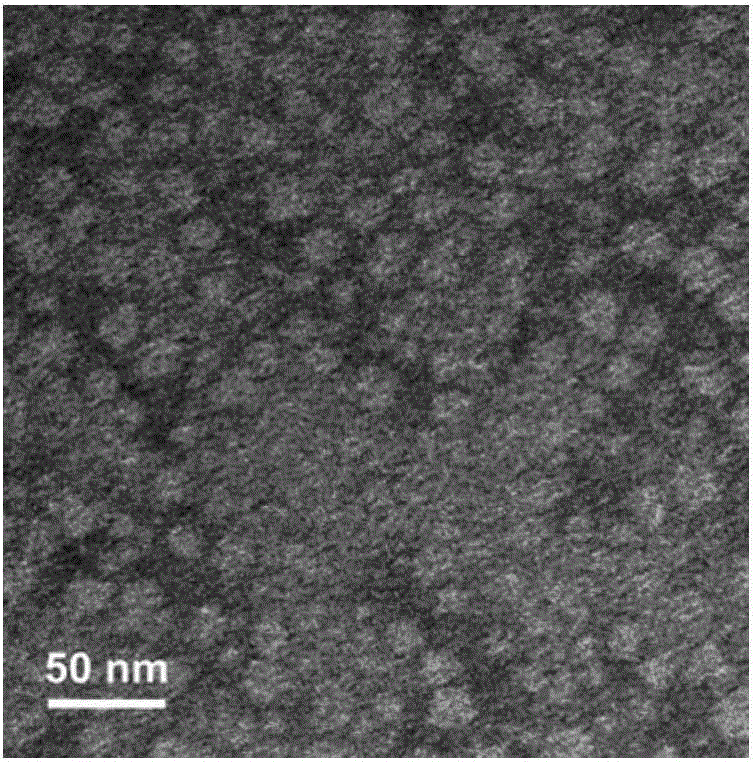
[0090] Dilute the micellar E7 vaccine solution prepared in Example 1 with deionized water to dilute the PEG-PE concentration to 0.1 mg / mL, take 10 μL of the sample solution, and add it dropwise to the carbon-coated surface treated with glow discharge hydrophilization After 30 seconds of adsorption on the membrane copper grid, absorb the sample solution with filter paper, add 10 μL of uranyl acetate (concentration: 2% (w / v)) dropwise and stain for 30 seconds, absorb the staining solution with filter paper, and use a transmission electron microscope (Tectai20 ) to observe the micelle morphology after negative staining. The result is as figure 1 As shown, the micellar vaccine was observed by transmission electron microscope to be uniform and spherical nanoparticles.
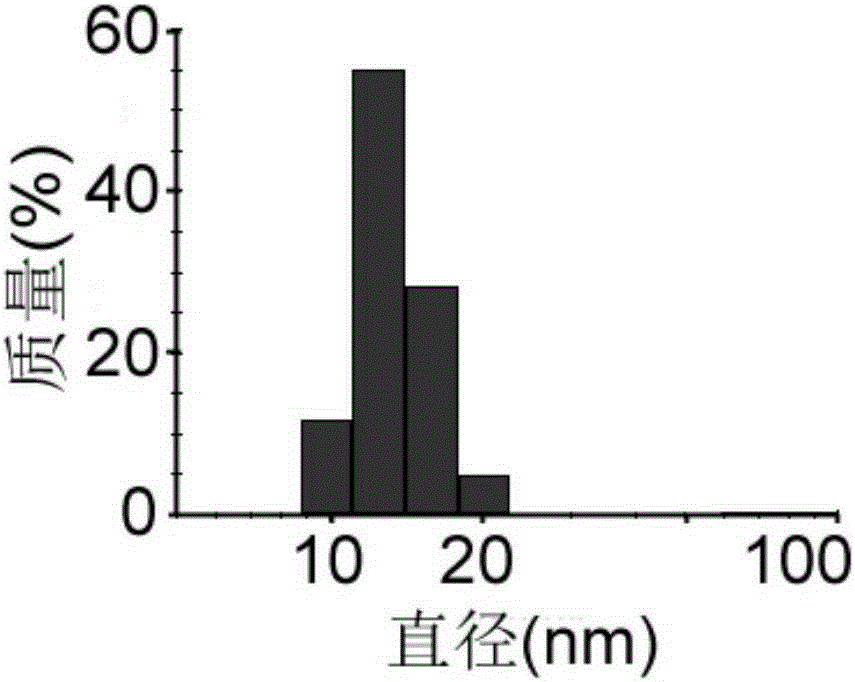
[0091] 2. Particle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com