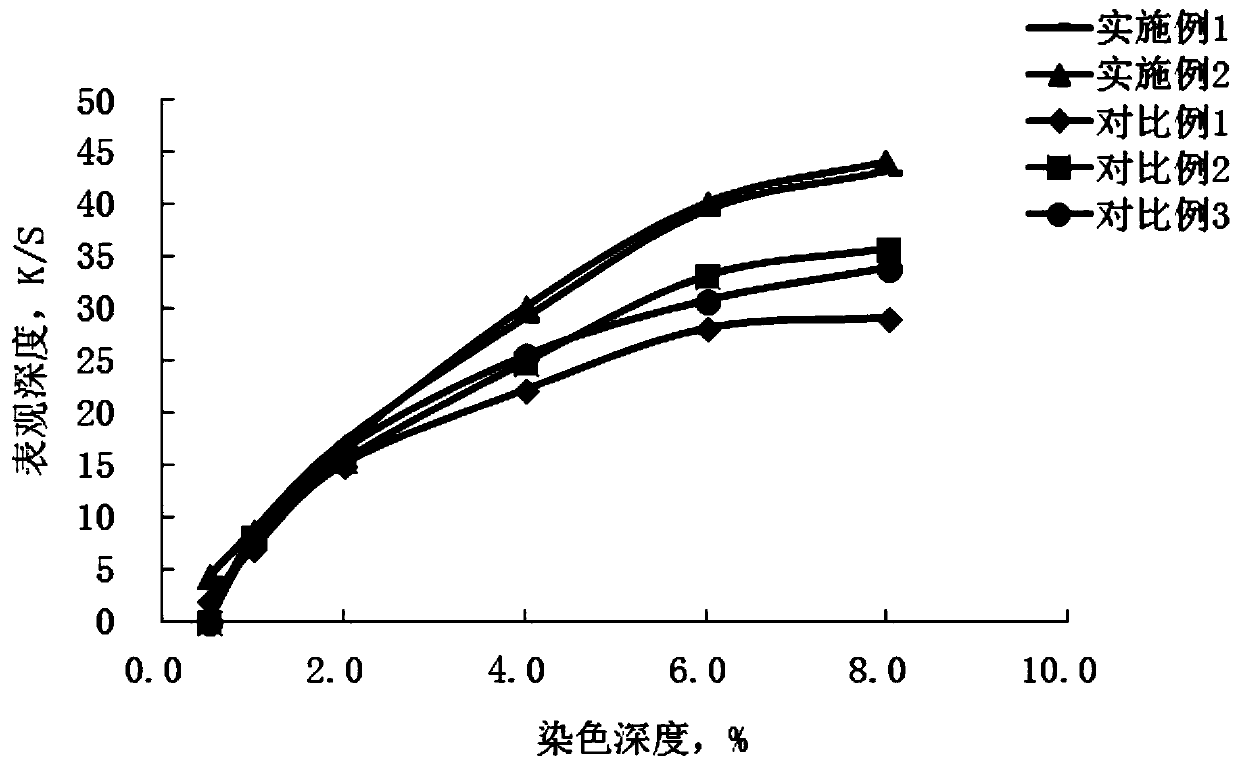
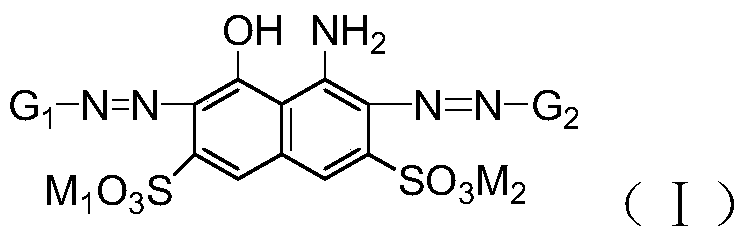
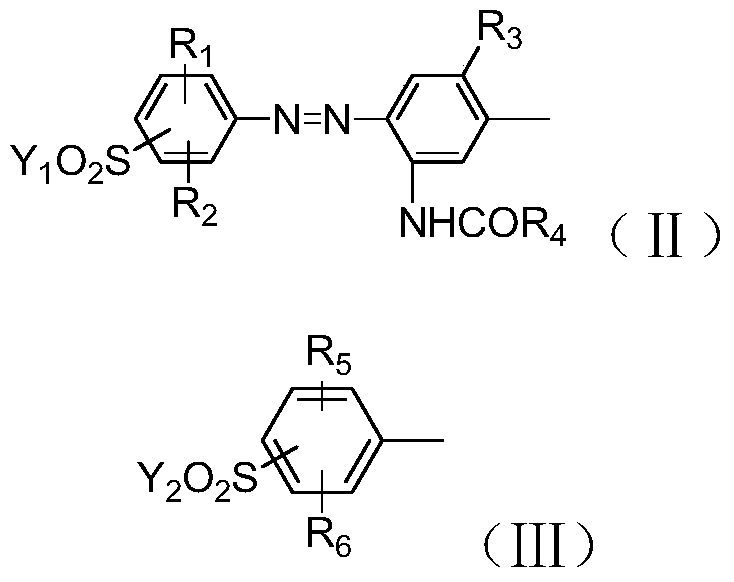
A kind of polyazo reactive dye compound and preparation method thereof
A technology of reactive dyes and compounds, which is applied in the field of polyazo reactive dye compounds and their preparation, can solve the problems of poor washing and rubbing fastness, unsatisfactory lifting power and dyeing depth, etc. Outstanding rubbing fastness, significant economic and social benefits, and excellent color fastness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]In a 1000ml beaker, add 150g of water and 36.1g of sulfonated para-ester (II-1), stir and beat for 1h, add 23g of 30% sodium nitrite solution at 10-15°C to carry out diazotization reaction for 2h, until the end point Finally, add sulfamic acid to destroy the excess nitrous acid, then add 15.1g m-ureidoaniline (II-2), control pH3~5 with baking soda, react at 10~15°C for 4~6h, and detect the disappearance of the diazonium salt. end. Adjust the pH of the reaction solution to 6.5-7 to make it completely dissolved, add 23g of 30% sodium nitrite solution, mix well, add the mixed solution to 30g of 30% hydrochloric acid and carry out diazotization reaction at 10-15°C 2 hour, add sulfamic acid to destroy excess nitrous acid after reaching the end point, obtain the first diazonium liquid, and set aside.
[0046] In a 1000ml beaker, add 150g of ice water and 28.1g of para-ester (Ⅲ-1), stir and beat for 1 hour, add 30g of 30% hydrochloric acid, and add 23g of 30% sodium nitrite so...
Embodiment 2
[0050] According to the preparation method described in Example 1, change 15.1g m-ureidoaniline into 15.0g m-acetamidoaniline to obtain the compound of formula (I-2), λmax=640nm in the aqueous solution, when applied to dyeing, the The fabric is dyed green.
[0051]
Embodiment 3~19
[0053]
[0054] According to the preparation method described in Example 1, the difference is that (II-1), (II-2) and (III-1) are replaced with the raw materials in the following Table 1 to obtain the dye compounds shown in the table, Can be used to dye fabrics green when applied to dyeing:
[0055]
[0056]
[0057]
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com