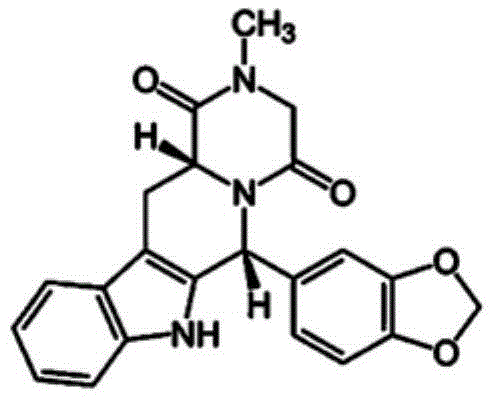
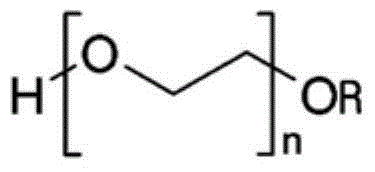Tadalafil free base-containing film dosage form containing polyethylene glycol-based polymer and/or vinyl pyrrolidone-based polymer as dispersion stabilizer
A technology of vinylpyrrolidone and polyethylene glycol, which is applied in the field of film preparation and its preparation, can solve problems such as layer separation and uneven active ingredients, and achieve the effect of reducing the amount of bubbles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
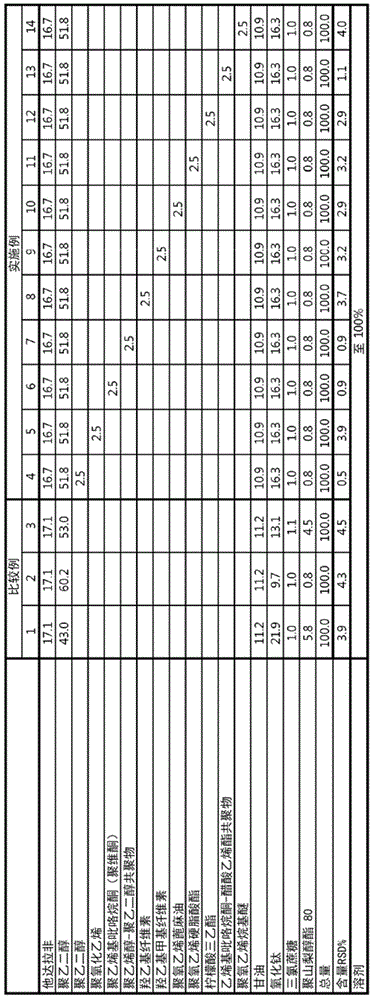
Examples
preparation example Construction
[0047] Preparation of film formulation containing tadalafil free base >
[0048] A film formulation containing tadalafil free base was prepared as follows. Plasticizers, additives, sweeteners, surfactants, and dispersion stabilizers are added to purified water, stirred until dissolved or dispersed in the purified water, and tadalafil free base is added thereto. Subsequently, homogenization was performed using a homogenizer (Ultraturrax T-25, IKA). Add polymer to it and homogenize with the same homogenizer. Then, the gas was removed from the film preparation solution under vacuum at 45° C., and after cooling to room temperature, it was coated with a polyethylene (PE) film of the most suitable thickness. Then, drying was performed at 80°C to prepare a film formulation containing tadalafil free base.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com