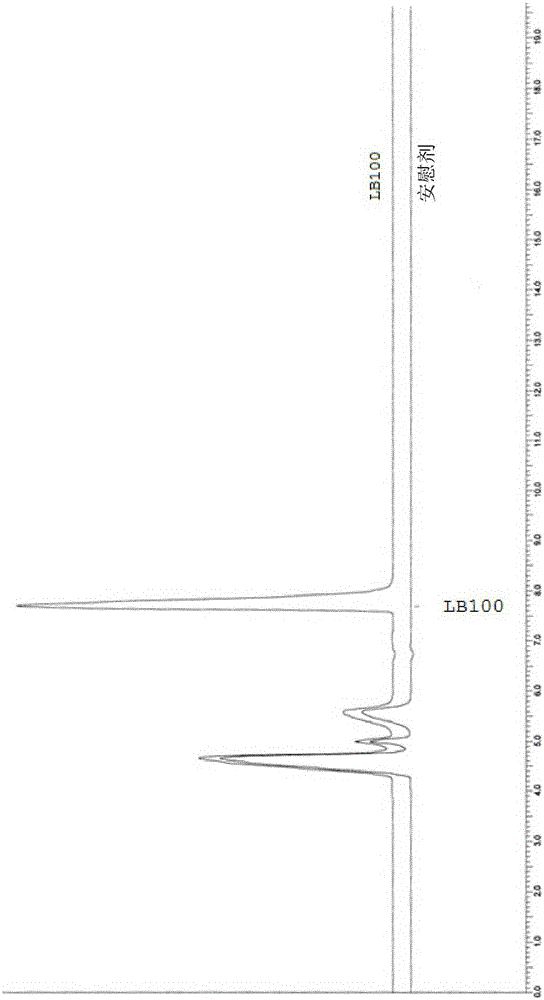
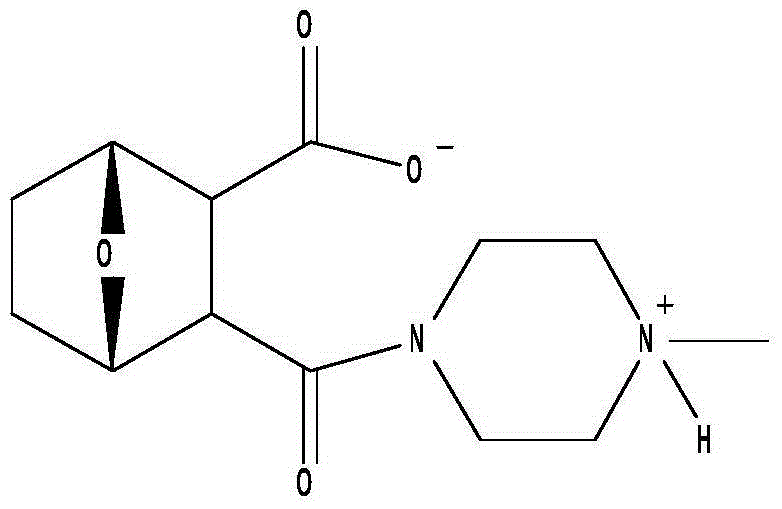
Formulations of oxabicycloheptanes and oxabicycloheptenes
A technology of alkyl and alkenyl, applied in the field of No. 565, No. WO2010/0
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0382] Stability study of example 1.LB-100 in normal saline and sodium bicarbonate
[0383] 1.1 Purpose
[0384] To determine the stability of LB-100 stored at room temperature and under refrigeration in saline and 4.2% sodium bicarbonate formulations.
[0385] 1.2 Materials and methods
[0386] 1.2.1 Preparations
[0387] LB-100 (Ash Stevens, Inc., Riverview, MI) was stored refrigerated and is considered stable under such storage conditions.
[0388] The vehicles used to prepare the LB-100 formulation were normal saline (0.9% Sodium Chloride Injection USP) (Baxter, Deerfield, IL) and 4.2% Sodium Bicarbonate, which Prepared by diluting 8.4% Sodium Bicarbonate Injection (Seneca Medical, Tiffin OH) 2-fold with Milli-Q water.
[0389] The saline formulation and the 4.2% sodium bicarbonate formulation were each prepared as follows at a target LB-100 concentration of 1.00 mg / mL. Weigh approximately 20 mg LB-100 into a tared glass vial. Vehicle is added to obtain the desired...
example 2
[0398] Example 2. Stability of LB-100 in glutamate, triethanolamine and phosphate buffer
[0399] 2.1 Purpose
[0400] To compare the long-term storage stability of LB-100 in the following formulations:
[0401] 1mg / mLLB-100 in glutamic acid buffered saline, pH8.5±0.1;
[0402] 1mg / mLLB-100 in glutamate buffer, pH10.5±0.1;
[0403] 1mg / mLLB-100 in triethanolamine buffer, pH7.0±0.1;
[0404] 1 mg / mL LB-100 in triethanolamine buffer, pH 9.0±0.1; and
[0405] 1mg / mLLB-100, in phosphate buffer, pH8.0±0.1.
[0406] 2.2 Materials and methods
[0407] 2.2.1 Preparations
[0408] 2.2.1.10.1 M glutamate solution
[0409] 28.1 g ± 0.1 g L-glutamate monosodium salt monohydrate was weighed out and added to 1500 mL nanopure water. The mixture was mixed until all the salts were dissolved to form a 0.1 mL stock solution of glutamic acid monosodium salt monohydrate.
[0410] Adjust the pH of 750 mL of the stock solution of L-glutamic acid monosodium salt monohydrate to 8.5 ± 0.1 us...
example 3
[0450] Example 3. Pharmaceutical compositions comprising LB-100
[0451] The following protocol was used to manufacture 42 L of a pharmaceutical composition comprising 1 mg / mL LB-100 and 0.1 M monosodium glutamate, pH 10.5.
[0452] 3.1 Materials
[0453] Table 11. Formulation ingredients
[0454]
[0455] Table 12. Container and Closure Components
[0456]
[0457] Table 13. Filtration and plumbing components
[0458]
[0459] 3.2 Preparation process
[0460] Add approximately 34kg of sterile water for injection USP to the 40L hyaluronic acid bottle. Sodium L-glutamate monohydrate was then added to the acid bottle and mixed for a minimum of ten minutes. The pH of the resulting mixture was adjusted to a pH in the range 10.4-10.6 with sodium hydroxide and / or hydrochloric acid. The target pH for this pH adjustment step is 10.5. Continue mixing the mixture until all the solids in the acid bottle have dissolved.
[0461] Next, add LB-100 to the acid bottle and m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com