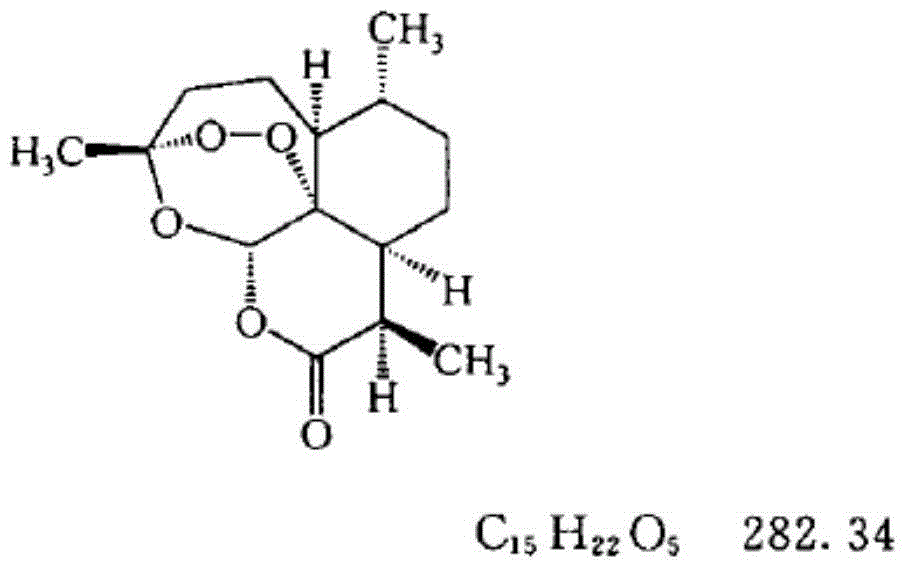
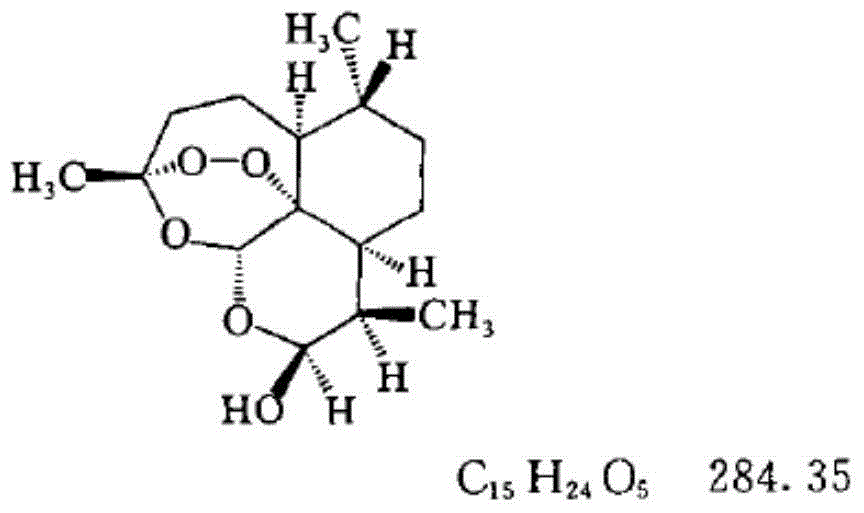
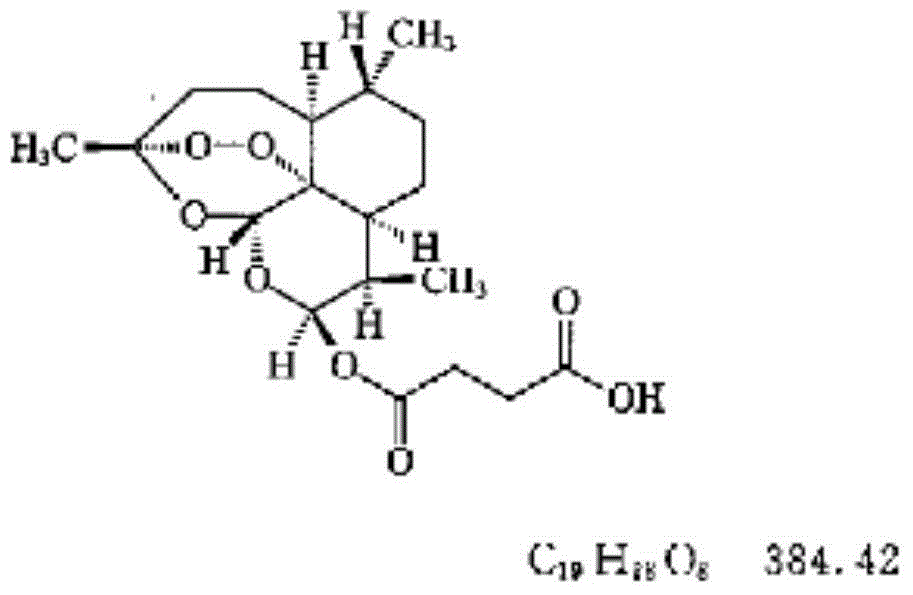
Medicated bath granules of artemisinin or derivatives of artemisinin and application of medicated bath granules
A derivative, artemisinin technology, applied in the field of medicated bath granules, can solve problems such as unsuitability for use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] The preparation of embodiment 1 artemisinin medicinal bath granule
[0121] Prescription 1:
[0122] Artemisinin 200g
[0123] Sodium bicarbonate 3750g
[0125] --------------------------------------
[0126] Make 1000 bags
[0127] Preparation method: In a D-level clean area that complies with the provisions of the Good Manufacturing Practice (GMP), the prescribed quantities of artemisinin, sodium bicarbonate, and sodium sulfate are respectively pulverized with a pulverizer, passed through a 60-mesh sieve, and then added with V -type mixer, mixed in mixer for 10 minutes, and the material after total mixing was bagged on the granule distributing machine, and the dispensing amount was 6.5g, made 1000 bags, promptly got artemisinin medicated bath granule (I).
[0128] Prescription 2:
[0129] Artemisinin 6250g
[0130] Sodium bicarbonate 10000g
[0131] Sodium sulfate 10000g
[0132] --------------------------------------
[0133] M...
Embodiment 2
[0142] The preparation of embodiment 2 dihydroartemisinin medicated bath granule
[0143] Prescription 4:
[0144] Dihydroartemisinin 80g
[0145] Sodium bicarbonate 3750g
[0146] Sodium sulfate 2550g
[0147] --------------------------------------
[0148] Make 1000 bags
[0149] Preparation method: In a D-level clean area that complies with the provisions of the Good Manufacturing Practice (GMP), the prescription quantities of dihydroartemisinin, sodium bicarbonate, and sodium sulfate are respectively pulverized with a pulverizer, passed through a 60-mesh sieve, and sequentially Add V-type mixer, mix in mixer for 10 minutes, the material after total mixing is bagged on the particle packing machine, and the subpackage is 7.1g, makes 1000 bags, obtains dihydroartemisinin medicated bath granule ( I).
[0150] Prescription 5:
[0151] Dihydroartemisinin 2000g
[0152] Sodium bicarbonate 8750g
[0153] Sodium sulfate 4250g
[0154] -------------------------------------...
Embodiment 3
[0164] The preparation of embodiment 3 artesunate medicated bath granules
[0165] Prescription 7:
[0166]Artesunate 300g
[0167] Sodium bicarbonate 3750g
[0168] Sodium sulfate 2550g
[0169] --------------------------------------
[0170] Make 1000 bags
[0171] Preparation method: In a D-level clean area that complies with the provisions of the Good Manufacturing Practice (GMP), the prescribed quantities of artesunate, sodium bicarbonate, and sodium sulfate are respectively pulverized with a pulverizer, passed through a 60-mesh sieve, and added in sequence V-type mixer, mixed in the mixer for 10 minutes, the material after the total mixing was bagged on the particle packing machine, and the sub-package was 6.6g, and 1000 bags were made to obtain the artesunate medicated bath granule (I) .
[0172] Prescription 8:
[0173] Artesunate 10000g
[0174] Sodium bicarbonate 10000g
[0175] Sodium sulfate 10000g
[0176] --------------------------------------
[0177]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com