Vilazodone orally disintegrating tablet and preparation method thereof
A technology for oral disintegrating tablets and disintegrating agents, which can be applied in the field of medicine and can solve problems such as patent blanks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
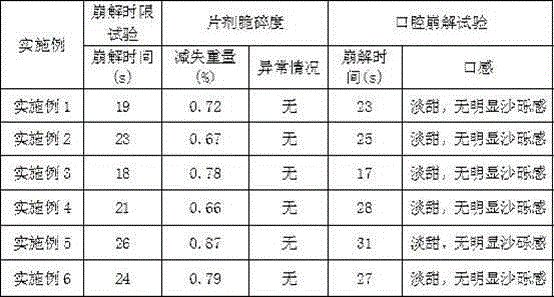
Embodiment 1
[0031] Example 1 Preparation of Vilazodone Orally Disintegrating Tablets (10mg)
[0032] Tablet composition Weight ratio (%) Vilazodone 10 Mannitol 40 microcrystalline cellulose 31.5 Crospovidone 8 Croscarmellose Sodium 8 Acesulfame K 1 silica 1 Magnesium stearate 0.5
[0033] Preparation Process:
[0034] (1) Crushing the vilazodone raw material through a 100-mesh sieve;
[0035] (2) Mix the pulverized raw materials with the prescribed amount of mannitol, microcrystalline cellulose, crospovidone, croscarmellose sodium, and acesulfame potassium;
[0036] (3) Add silicon dioxide and magnesium stearate to the above mixture, mix well and press into tablets with a weight of 100mg and a hardness of 30-40N.
Embodiment 2
[0037] Example 2 Preparation of Vilazodone Orally Disintegrating Tablets (20mg)
[0038] Tablet composition Weight ratio (%) Vilazodone 20 Mannitol 40 microcrystalline cellulose 30.5 Crospovidone 8 Croscarmellose Sodium 8 Acesulfame K 2 silica 1 Magnesium stearate 0.5
[0039] Preparation Process:
[0040] (1) Crushing the vilazodone raw material through a 100-mesh sieve;
[0041] (2) Mix the pulverized raw materials with the prescribed amount of mannitol, microcrystalline cellulose, crospovidone, croscarmellose sodium, and acesulfame potassium;
[0042] (3) Add silicon dioxide and magnesium stearate to the above mixture, mix well and press into tablets with a tablet weight of 110mg and a hardness of 30-40N.
Embodiment 3
[0043] Example 3 Preparation of Vilazodone Orally Disintegrating Tablets (10mg)
[0044] Tablet composition Weight ratio (%) Vilazodone 10 Woodnitol 42 microcrystalline cellulose 31 Crospovidone 6 Croscarmellose Sodium 8 Acesulfame K 1 aspartame 0.5 silica 1 Magnesium stearate 0.5
[0045] Preparation Process:
[0046] (1) Crushing the vilazodone raw material through a 100-mesh sieve;
[0047] (2) Add and mix the pulverized raw materials with the prescribed amount of woodnitol, microcrystalline cellulose, crospovidone, croscarmellose sodium, acesulfame potassium, and aspartame ;
[0048] (3) Add silicon dioxide and magnesium stearate to the above mixture, mix well and press into tablets with a weight of 100mg and a hardness of 30-40N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com