Celecoxib capsules and production technology thereof
A technology for celecoxib and production process, applied in the field of celecoxib capsules and production process thereof, can solve the problems of low dissolution rate, difficult absorption and the like, and achieve the effects of high dissolution rate, easy absorption and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
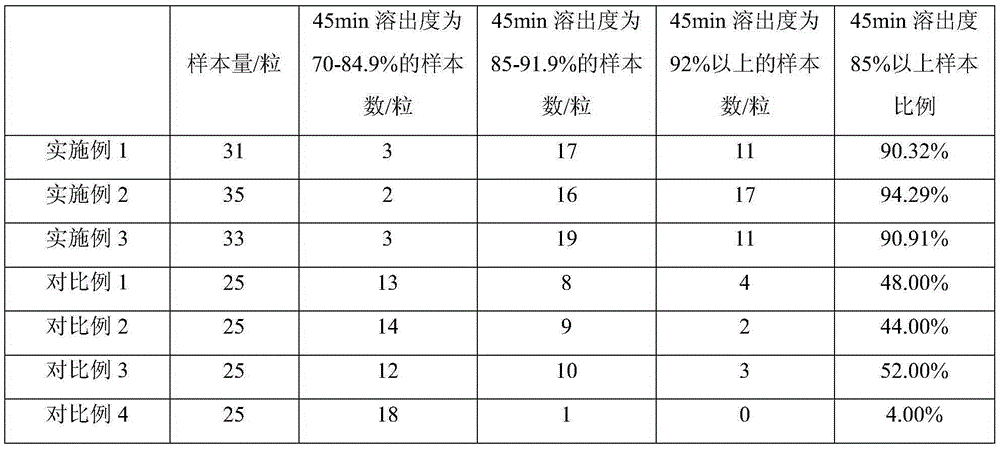
[0066] A celecoxib capsule comprising the following raw materials in mass percentage: celecoxib: 65.43%; lactose monohydrate: 25.31%; povidone: 4.07%; croscarmellose sodium: 0.56%; Sodium Dialkyl Sulfate: 4.07%; Magnesium Stearate: 0.56%. The batch (8.10kg) production prescription is as follows: Celecoxib 5.3kg, lactose monohydrate 2.05kg, povidone 0.33kg, croscarmellose sodium 0.045kg, sodium lauryl sulfate 0.33kg, stearin Magnesium acid 0.045kg.
[0067] The production steps are as follows:
[0068] (1) Material distribution: Weigh each material in the weighing room according to the batch prescription, put them into PE bags, and put them into stainless steel barrels, and place them together.
[0069] (2) Preparation of granulation solution: add deionized water into a stainless steel container, stir until a vortex is formed, slowly add sodium lauryl sulfate, continue stirring for more than 15 minutes at a speed that can form a vortex, and disperse until it is completely dis...
Embodiment 2
[0083] A celecoxib capsule comprising the following raw materials in mass percentage: celecoxib: 74.07%; lactose monohydrate: 18.43%; povidone: 2.5%; croscarmellose sodium: 1%; Sodium Dialkyl Sulfate: 3%; Magnesium Stearate: 1%. The batch (8.10kg) production prescription is as follows: celecoxib 6.00kg, lactose monohydrate 1.50kg, povidone 0.20kg, croscarmellose sodium 0.08kg, sodium lauryl sulfate 0.24kg, stearin Magnesium acid 0.08kg.
[0084] The production steps are basically the same as in Example 1, and the detection steps are also basically the same as in Example 1.
Embodiment 3
[0086] A celecoxib capsule comprising the following raw materials in mass percentage: celecoxib: 84.57%; lactose monohydrate: 12.22%; povidone: 1.05%; croscarmellose sodium: 0.56%; Sodium Dialkyl Sulfate: 1.05%; Magnesium Stearate: 0.56%. The batch (8.10kg) production prescription is as follows: Celecoxib 6.85kg, lactose monohydrate 0.99kg, povidone 0.085kg, croscarmellose sodium 0.045kg, sodium lauryl sulfate 0.085kg, stearin Magnesium acid 0.045kg.
[0087] The production steps are basically the same as in Example 1, and the detection steps are also basically the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com