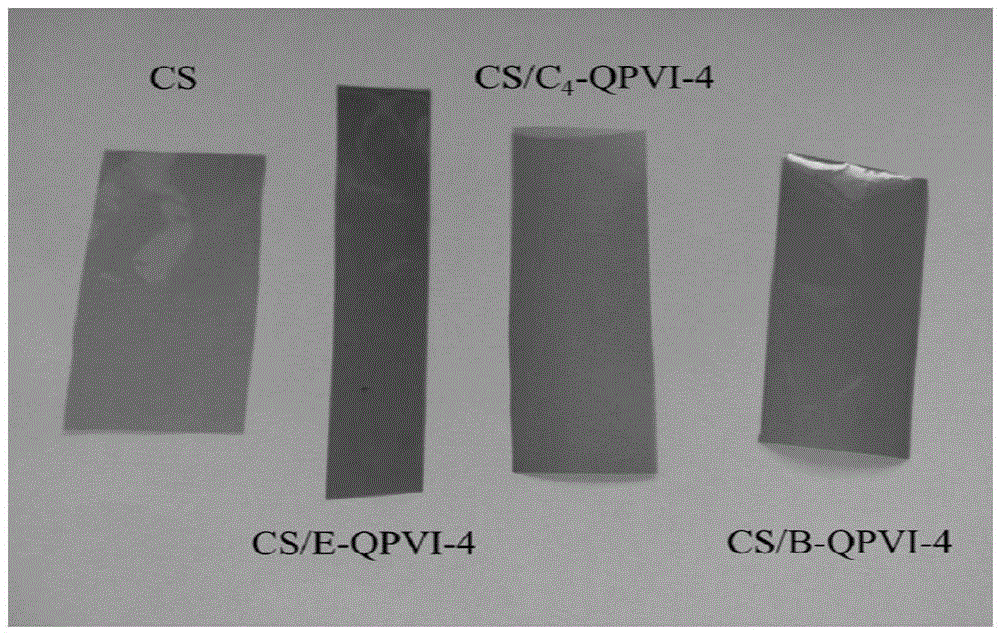
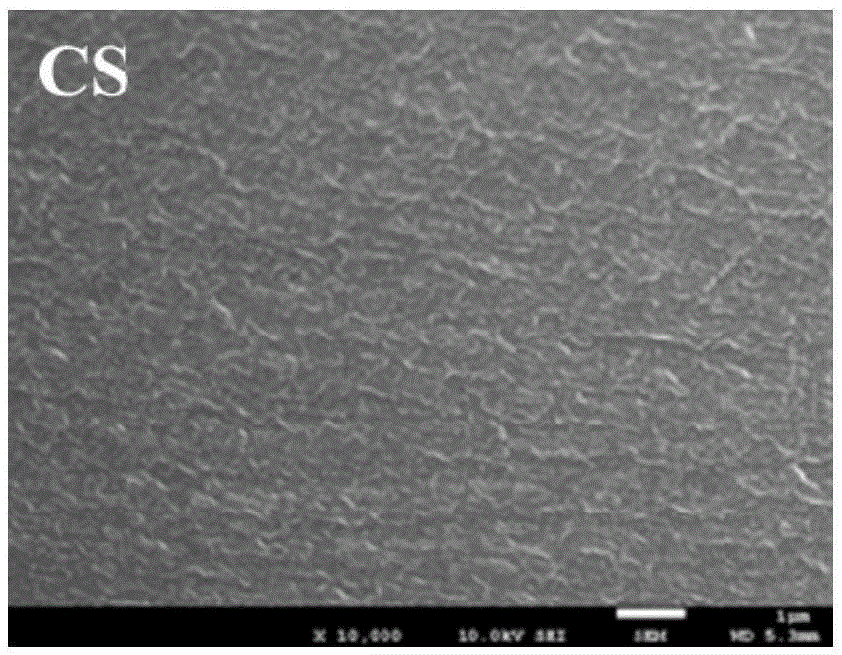
Organic nano-composite anion exchange membrane and preparation method therefor and application thereof
An anion exchange membrane and nanocomposite technology, applied in the field of organic nanocomposite anion exchange membrane and its preparation, to achieve the effect of high conductivity and good mechanical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Disperse 0.6mL of N-vinylimidazole, 0.6mL of ethylene glycol dimethacrylate and 0.018g of azobisisobutyronitrile into 80mL of acetonitrile, heat to reflux at 82°C, and centrifuge and wash the obtained product with acetonitrile for 2 times, and then centrifuged and washed once with absolute ethanol to obtain imidazole nanospheres.
[0033] Disperse 0.6 g of prepared imidazole nanospheres and a quaternization reagent (2.40 mL of ethyl chloroformate, 1.32 mL of n-chlorobutane, and 1.48 mL of benzyl chloride) into absolute ethanol (120 mL), Heated to reflux for 20 h at 70°C under nitrogen protection. The solution after the reaction was centrifuged and washed 3 times with absolute ethanol, and dried in a vacuum oven for 24 hours to obtain E-QPVI, C 4 -QPVI, B-QPVI quaternized nano-microspheres.
[0034] During the preparation of the organic nanocomposite membrane, quaternized imidazole nanospheres (0.048 g) were added to 10 mL of deionized water, and stirred ultrasonically...
Embodiment 2
[0039] Disperse 0.6mL of N-vinylimidazole, 0.6mL of ethylene glycol dimethacrylate and 0.018g of azobisisobutyronitrile into 80mL of acetonitrile, heat to reflux at 82°C, and centrifuge the obtained product with acetonitrile for 2 times, and then centrifuged and washed once with absolute ethanol to obtain imidazole nanospheres.
[0040] Disperse 0.6 g of prepared imidazole nanospheres and 2.4 mL of ethyl chloroformate into 120 mL of absolute ethanol, and heat to reflux for 20 h at 70°C under nitrogen protection. The solution after the reaction was centrifuged and washed 3 times with absolute ethanol, and dried in a vacuum oven for 24 hours to obtain E-QPVI quaternized imidazole nanospheres.
[0041] During the preparation process of the organic nanocomposite membrane, 0.024 g of E-QPVI was added into 10 mL of deionized water, and stirred ultrasonically (ultrasonic time was greater than 4 h). Add 1.2 g of chitosan to 45 mL of deionized water, add 1 mL of glacial acetic acid to...
Embodiment 3
[0045] Disperse 0.6mL of N-vinylimidazole, 0.6mL of ethylene glycol dimethacrylate and 0.018g of azobisisobutyronitrile into 80mL of acetonitrile, heat to reflux at 82°C, and centrifuge the obtained product with acetonitrile for 2 times, and then centrifuged and washed once with absolute ethanol to obtain imidazole nanospheres.
[0046] Disperse 0.6 g of prepared imidazole nanospheres and 2.4 mL of ethyl chloroformate into 120 mL of absolute ethanol, and heat to reflux for 20 h at 70°C under nitrogen protection. The solution after the reaction was centrifuged and washed 3 times with absolute ethanol, and dried in a vacuum oven for 24 hours to obtain E-QPVI quaternized imidazole nanospheres.
[0047] During the preparation of the organic nanocomposite membrane, E-QPVI (0.72 g) was added to 10 mL of deionized water and stirred ultrasonically (ultrasonic time was greater than 4 h). Add 1.2 g of chitosan to 45 mL of deionized water, add 1 mL of glacial acetic acid to the chitosan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Young's modulus | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com