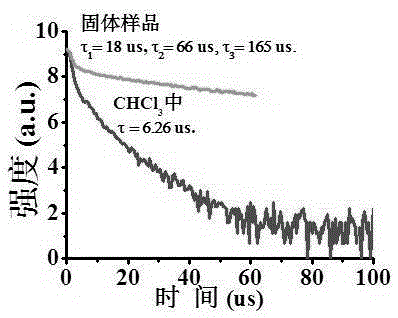
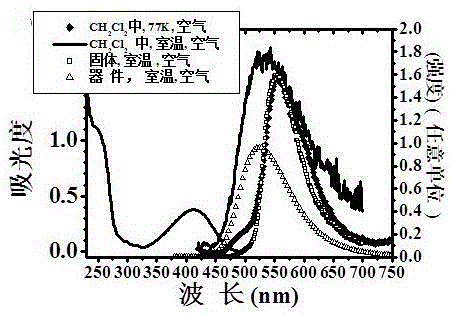
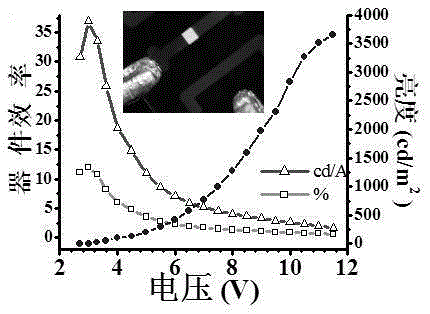
Method for generating phosphorescence in organic compound
A technology of organic compounds and phosphorescence, applied in the fields of organic chemistry, chemical instruments and methods, luminescent materials, etc., which can solve the problems of limited classical concepts, failure to find the fundamental mechanism of phosphorescence, acceleration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] A synthetic method route of an organic material (BDDO) is:
[0031] ,
[0032] Specific steps are as follows:
[0033] (1), use 1,4-dibromo-2,3,5,6-tetrabromomethylbenzene as raw material to synthesize 1,4-dibromo-2,3,5,6-tetramethoxybenzene, put 1,4-dibromo-2,3,5,6-tetrabromomethylbenzene is added to methanol solution to dissolve an equivalent amount of metal sodium, and after sodium methoxide is produced, alcoholysis is performed to obtain 1,4-dibromomethylbenzene Bromo-2,3,5,6-tetramethoxybenzene, which can be easily oxidized;
[0034] (2) Oxidation of 1,4-dibromo-2,3,5,6-tetramethoxybenzene by alkaline potassium permanganate as raw material to obtain 3,6-dibromobenzene-1,2,4,5 -Tetracarboxylic acid, the molar ratio of potassium permanganate to potassium hydroxide is 6:1, the ratio of potassium permanganate to 2 is the ratio of redox number, add potassium permanganate several times, and heat before the first addition , to prevent splashing caused by the accumul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com