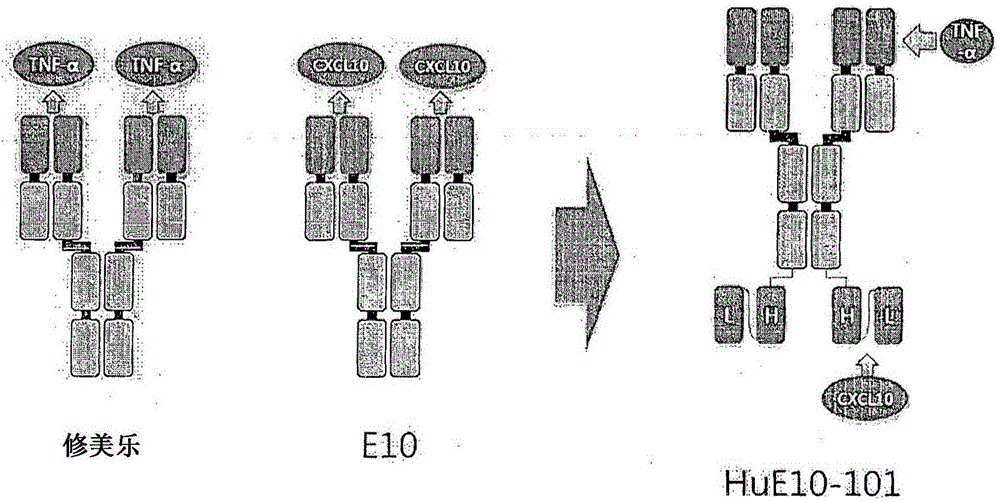
Anti-tnf- alpha /cxcl10 double-targeting antibody and use thereof
A double-targeted antibody, antibody technology, applied in the direction of antibodies, antibody medical components, anti-animal/human immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Embodiment 1: the production of human CXCL10 antigenic protein
[0137] To construct monoclonal antibodies against human CXCL10, human CXCL10 protein was expressed and purified. Specifically, to amplify human CXCL10, a mixture of spleen, placenta, liver, and kidney cDNA libraries was used as a template, which was inserted into pET22b vector. BL21(DE3) was used to transform pET22b-human CXCL10, and the obtained transformant was inoculated on an LB plate containing ampicillin, cultivated overnight, and then cultured with shaking at 37°C.
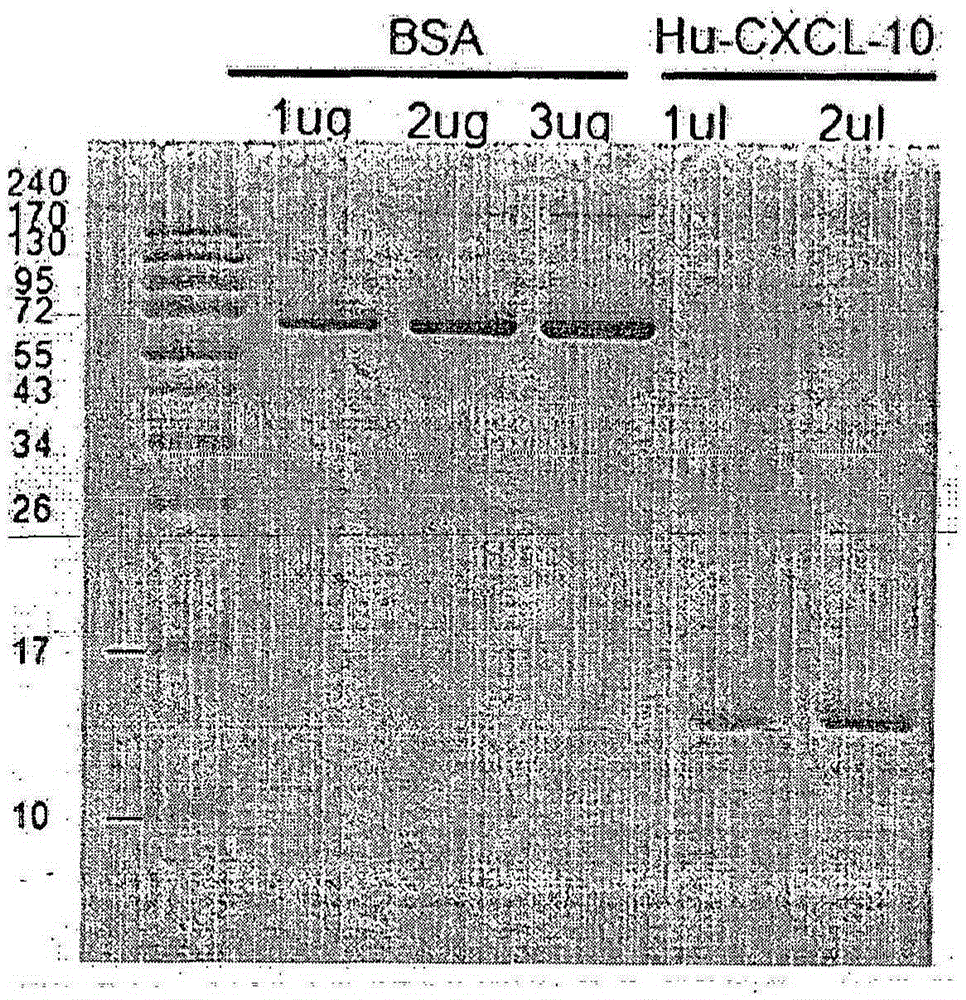
[0138] Thereafter, when the OD600 was 0.55, 0.5 mM IPTG was added to the cells, cultured overnight, and centrifuged at 6,500 rpm at 4° C. for 15 minutes to obtain a supernatant. The obtained supernatant was concentrated and reacted with Ni-NTA agarose, and then eluted by packing the column with agarose beads. After concentration, the resulting product was loaded onto a 15% SDS-PAGE gel and stained with Coomassie blue. To compare the ...
Embodiment 2
[0139] Embodiment 2: the construction of monoclonal antibody
[0140] 2-1. Panning process
[0141] Panning is the process of selecting only phage-displayed peptides on their surface that have the property of binding to target molecules (antibodies, enzymes, cell surface receptors, etc.) from a phage library displaying peptides on the phage capsid . To construct a phage antibody panel for the construction of monoclonal antibodies against CXCL10, phage panning was performed. 100 μg of the purified human CXCL10 antigen obtained in Example 1 was used in 2 ml of coating buffer (Na 2 CO 3 (Sigma, S7795) 1.59gNaHCO 3 (Sigma, S8875) 2.93gNaN 3 (Sigma, S2002), 0.2g) was coated at 4°C for about 16 hours, and diluted in PBS at room temperature for 2 hours, and then immunized with 4% skimmed milk ((BD, 232100)-4%, in 1XPBS) Reaction blocked in sorbent tube. 2 ml of phage library was added to the immunosorbent tube, allowed to react at room temperature for 2 hours, and then washed ...
Embodiment 3
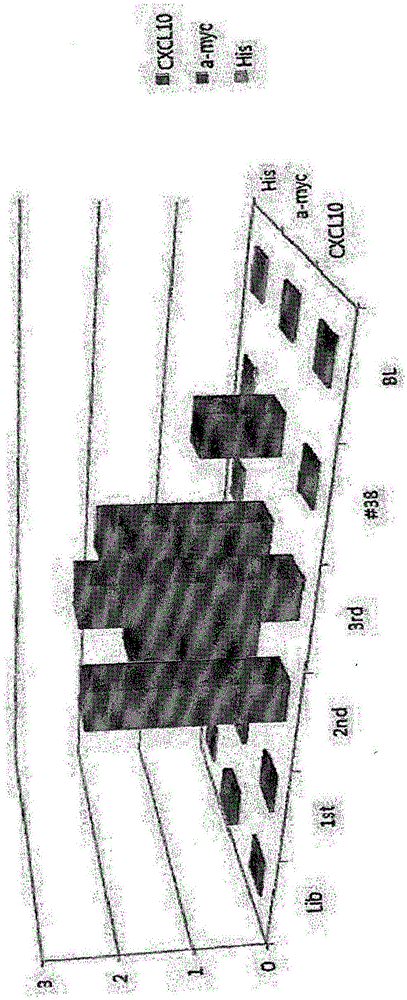
[0156] Example 3: Classification and Research of Selected Monoclonal Phages
[0157] 3-1. Verification by fingerprint analysis
[0158] In order to verify the 10 monoclonal cells selected in Example 2 by fingerprint analysis, 1 μl of the selected monoclonal cells were mixed with 0.2 μl of TaqDNA polymerase (Gendocs, 5 U / μl), 50 p / μl of forward primer ( pelB5) (SEQ ID NO:41:5'-CTAGATAACGAGGGCAAATCATG-3') and reverse primer (cla3) (SEQ ID NO:42:5'-CGTCACCAATGAAACCATC-3') 0.2 μl each, 3 μl of 10X buffer and 0.6 μl of 10 mM dNTP mix and 24.8 μl of distilled water were mixed, and clone PCR (iCycleriQ, BIO-RAD) was performed under the following conditions for the PCR program shown in Table 3.
[0159] 【table 3】
[0160]
[0161] The clone PCR product thus obtained was identified on 1% agarose gel (SeakemLE, CAMERES50004), and 0.2 μl of BstNI (Roche 11288075001, 10 U / μl) was added to the PCR product, and it was heated at 37° C. React under the reaction conditions for 2 to 3 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Recovery rate | aaaaa | aaaaa |
| Total molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com