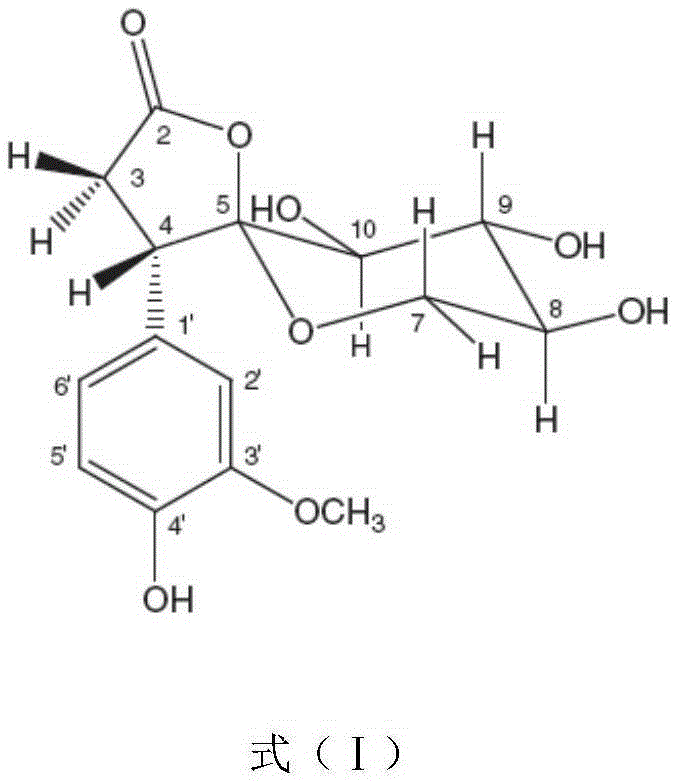
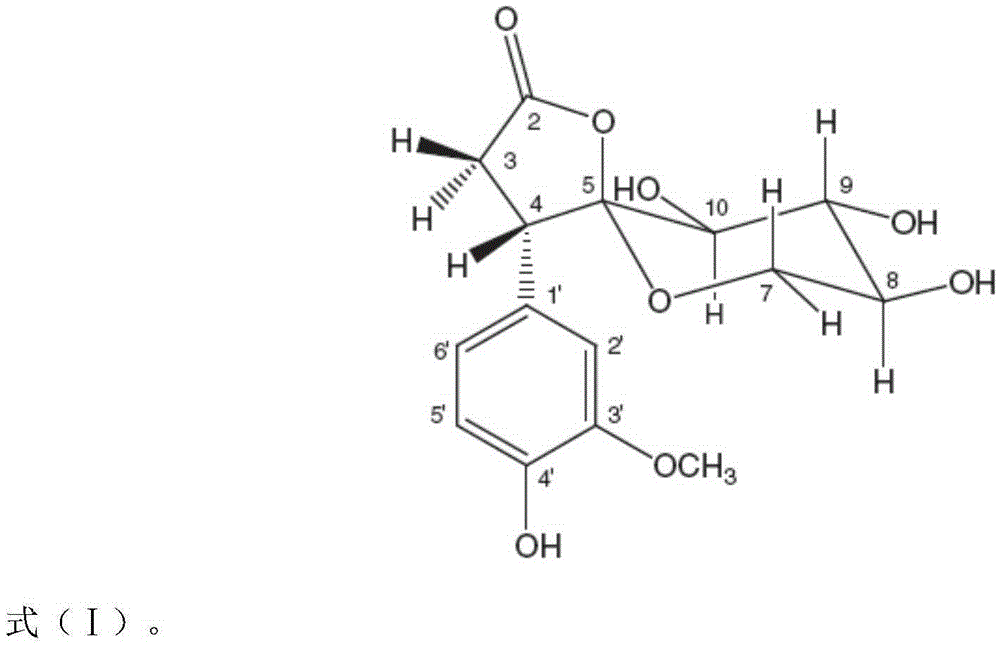
Application of Foveospirolide in preparation of tubercle bacillus treating and resisting medicine
An anti-tuberculosis and drug technology, applied in antibacterial drugs, organic chemistry and other directions, can solve the problems of difficult tuberculosis prevention and treatment, and the treatment and management of tuberculosis patients is not very standardized, and achieves strong tuberculosis inhibitory activity, outstanding substantive characteristics, The effect of widening the variety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the preparation of the compound Foveospirolide tablet involved in the present invention:
[0016] Get 20 grams of compound Foveospirolide, add 180 grams of conventional excipients for tablet preparation, mix well, and make 1000 tablets with a conventional tablet press.
Embodiment 2
[0017] Embodiment 2: the preparation of compound Foveospirolide capsules involved in the present invention:
[0018] Get 20 grams of compound Foveospirolide, add conventional auxiliary materials for preparing capsules such as 180 grams of starch, mix well, and make 1000 capsules.
experiment example 1
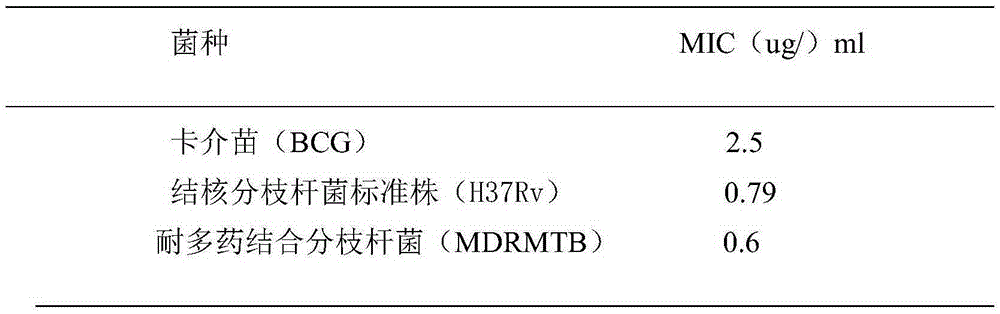
[0019] Experimental Example 1 Solid Medium Dilution Method Determination of Foveospirolide Anti-Bacillus Calmette-Guerin (BCG) Absolute Concentration
[0020] Scrape the BCG culture from the inclined surface, add it to 3ml Middlebrook7H9 broth medium, add a small amount of glass beads, tighten the test tube cap, vibrate and grind vigorously on the vortex oscillator, and compare it with the standard McFarland turbidimetric tube (MacFarland No.1). Turbidity, that is to make 1mg / ml bacillus Calmette-Guerin (BCG) bacterial suspension.
[0021] Foveospirolide is made into high-concentration stock solution with DMSO, dilutes stock solution to required concentration with 5% Tween-80 sterile ultrapure water, and the diluted Foveospirolide is added to 4mlMiddlebrook7H11 agar medium by required dosage (this culture Base has been sterilized by high pressure steam at 121°C for 15 minutes, cooled to 50-55°C), mixed evenly to make Foveospirolide, the concentrations are 6.0ug / ml, 4.0ug / ml, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com