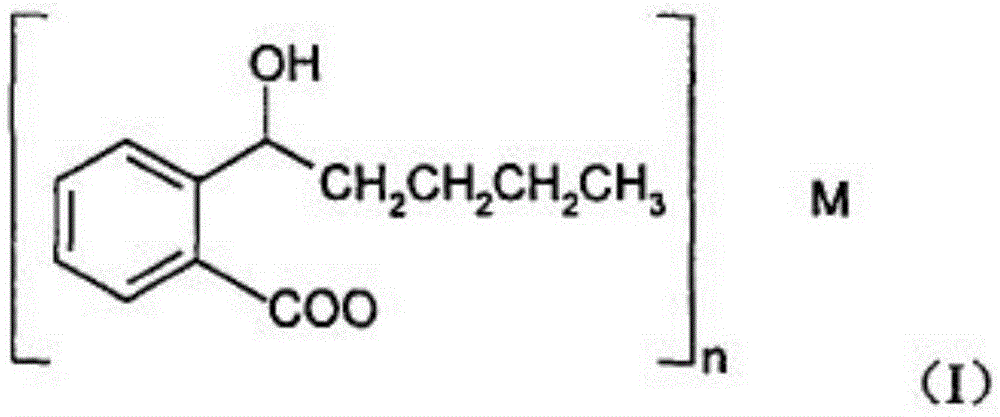
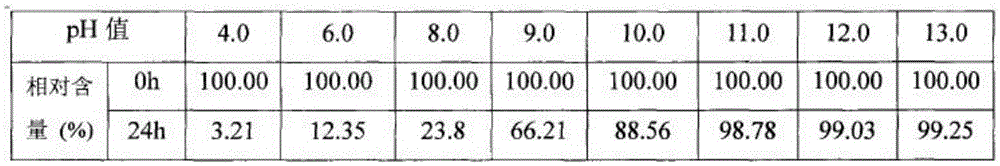
Racemic 2-(alpha-hydroxy amyl) benzoate freeze-dried powder injection and preparation method thereof
一种羟基戊基、苯甲酸盐的技术,应用在医药领域,能够解决易发生降解、稳定性差等问题,达到解决质量不稳定、稳定性增加、储运和使用方便的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Prescription composition (1000 sticks, 25mg / stick)
[0036] Racemic 2-(α-hydroxypentyl)sodium benzoate 25g
[0038] Anhydrous disodium hydrogen phosphate 3g
[0039] Preparation method: dissolve the excipients in water for injection, adjust the pH value to 12.0 with aqueous sodium hydroxide solution after dissolving; add the main drug to the above excipient solution with a pH value of 12.0 to dissolve, add water for injection, and adjust the pH value of the solution with sodium hydroxide solution to 12.0, with a final volume of 1000mL; sterilized by passing through a 0.22μm microporous membrane; filling, 1ml / bottle; semi-pressed butyl rubber stoppers, and freeze-drying in a freeze dryer: put the filled medicinal solution in - Pre-freeze at 35°C, and when the temperature of the liquid medicine reaches -35°C, maintain it for 4 hours; raise the temperature to -30°C in 4 hours, maintain it for 20 hours, and keep the vacuum degree <20Pa; raise ...
Embodiment 2
[0041] Prescription composition (1000 sticks, 25mg / stick)
[0042] Racemic 2-(α-hydroxypentyl)sodium benzoate 25g
[0044] Anhydrous disodium hydrogen phosphate 5g
[0045] Preparation method: with embodiment 1.
Embodiment 3
[0047] Prescription composition (1000 sticks, 25mg / stick)
[0048] Racemic potassium 2-(α-hydroxypentyl)benzoate 25g
[0050] Anhydrous disodium hydrogen phosphate 6g
[0051] Preparation method: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com