Targeting the EGFR-SGLT1 Interaction for Cancer Therapy
A cancer cell, composition technology, applied in the field of EGFR-SGLT1 interaction for targeted cancer therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
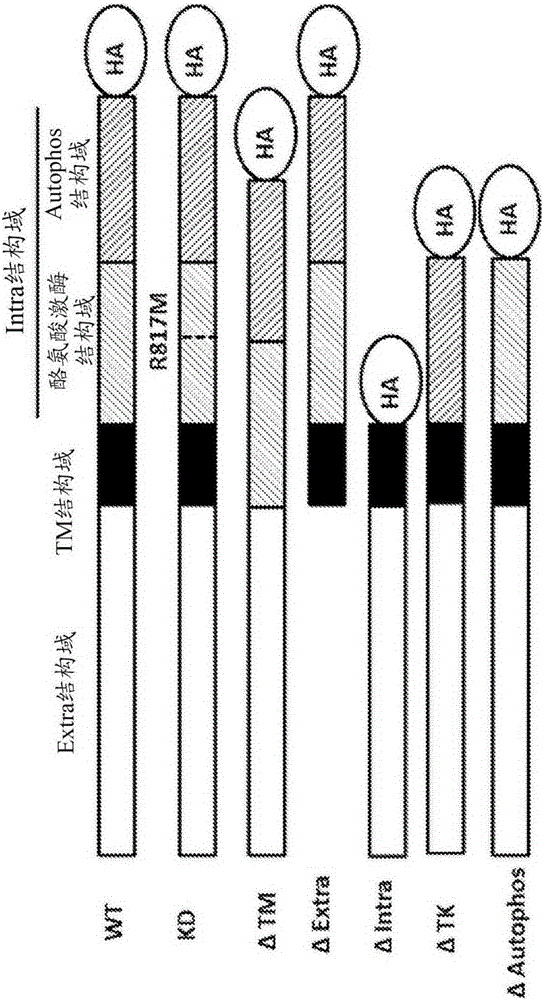
[0026] The key microdomain of EGFR, which is necessary for the full interaction and mutual stabilization of EGFR and SGLT1;
[0027] ■Activation / inactivation of EGFR on EGFR-SGLT1 interaction;
[0028] ■Measurement of EGFR and SGLT1 expression in prostate cancer tissues and cell lines;
[0029] Inhibitory effect of SGLT1 on the sensitivity of prostate cancer cells to EGFR tyrosine inhibitors;
[0030] ■ Amino acid sequence of the synthesized peptide ESD-01;
[0031] The effect of ESD-01 on the stability of EGFR and SGLT1 proteins in cancer cells; and
[0032] ■Effect of ESD-01 on the viability of non-cancer cells (HEK293) and several types of cancer cells cultured in vitro.
[0033] ■ ESD-01 peptide composed of L-amino acid or D-amino acid is also effective in killing cancer cells in vitro.
[0034] In one embodiment, the autophosphorylated region of EGFR (978-1210 amino acids) is required for adequate interaction of EGFR with SGLT1. This interaction is independent of the t...
Embodiment
[0058] cells and reagents. HEK293 cell line, prostate cancer cell line PC3, LNCaP, Du145, MDA-MB-231 and HCT116 cells were originally obtained from the American Type Culture Collection (ATCC) and cultured at 37 °C in 5% CO 2 , the cell lines were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin / streptomycin. Mouse anti-Flag-tag antibody (F1804), proteasome inhibitor MG231 and phlorizin dihydrate were obtained from Sigma-Aldrich (St. Louis, MO). AEE788, gefitinib and erlotinib were obtained from Selleckchem (Houston, TX). Antibody against pEGFR (Y1173) (Catalog #2434L) was obtained from Cell Signaling (Danvers, MA). Monoclonal antibody against C225 was obtained from Dr. Lee Elis (M.D. Anderson Cancer Center). Rabbit anti-actin (cat# sc-7210), rabbit anti-HA-tag antibody (sc-805), anti-rabbit and mouse secondary antibodies labeled with horseradish peroxidase, and protein A / G conjugate Agarose pellets (catalogue number sc-2003) were obtained from ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com