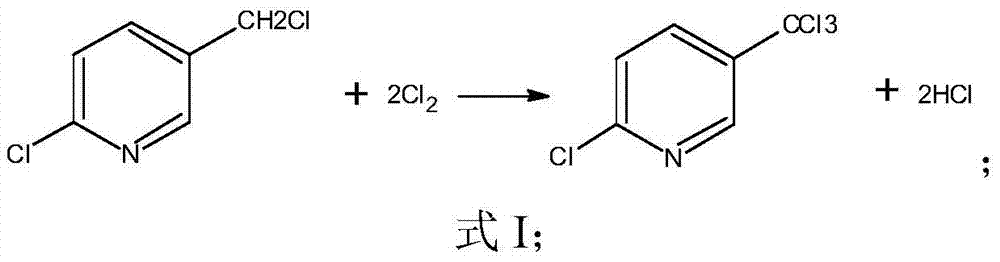
Industrial production method of 2-chloro-5-trichloromethyl pyridine
A technology of trichloromethylpyridine and chloromethylpyridine, which is applied in the industrial production field of 2-chloro-5-trichloromethylpyridine, and can solve the problems of long reaction time of catalyst, high reaction yield, influence on equipment productivity and the like , to avoid the darkening of the material color, increase the liquid phase chlorination temperature, and achieve the effect of fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 200kg of 2-chloro-5-chloromethylpyridine and 150kg of 4-chlorobenzotrifluoride to a 500L glass-lined reactor. After the reactor is heated to 120°C, chlorine gas is introduced, and ultraviolet light is turned on to initiate the reaction. Keep the reaction temperature at 120°C , after reacting for 30 hours, sample GC analysis, when 2-chloro-5-trichloromethylpyridine content ≥ 90.0%, stop chlorine gas feed; feed N 2 Until the tail gas is neutral, the temperature of the reaction kettle is lowered to 80°C, and put into the rectification kettle; under the conditions of a pressure of 2kpa, a temperature of the kettle of 110°C, a temperature of the top of the rectification tower of 64°C, and a reflux ratio of 6:1, the solvent is recovered, and the Under the conditions of 600Pa pressure, 160℃ kettle temperature, 114℃ rectification tower top temperature and 6:1 reflux ratio, 214kg of 2-chloro-5-trichloromethylpyridine was collected, with a content of 99.21%, and a melting poin...
Embodiment 2
[0029] Add 200kg of 2-chloro-5-chloromethylpyridine and 150kg of 3,4-dichlorobenzotrifluoride to a 500L glass-lined reactor. After the reactor is heated to 120°C, chlorine gas is introduced, and ultraviolet light is turned on to initiate the reaction. Reaction temperature to 160°C, keep 160°C for 12 hours, sample GC analysis, when the content of 2-chloro-5-trichloromethylpyridine is ≥90.0%, stop the introduction of chlorine gas; 2 Until the tail gas is neutral, the temperature of the reaction kettle is lowered to 80°C, and put into the rectification kettle; under the conditions of 2kpa pressure, 110°C kettle temperature, 87°C rectification column top temperature, and a reflux ratio of 6:1, the solvent is recovered, and the Under the conditions of 600Pa pressure, 160°C kettle temperature, 114°C rectification tower top temperature, and 6:1 reflux ratio, 243kg of 2-chloro-5-trichloromethylpyridine was collected with a content of 99.21% and a melting point of 53-55°C. The total yi...
Embodiment 3
[0031] Operate with embodiment 1, reaction temperature 80 ℃, reaction time 120hr. 202kg of 2-chloro-5-trichloromethylpyridine was obtained, with a content of 99.03% and a melting point of 53-55°C. The total yield is 70.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com