Preparation method of 2-chloroisonicotinate
A technology of chloroisonicotinic acid and dichloroisonicotinic acid, applied in organic chemistry and other directions, can solve the problems of harsh diazotization reaction conditions, reduced yield, long reaction period, etc., to avoid corrosion and environmental pollution, dechlorination The effect of mild conditions and short reaction times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
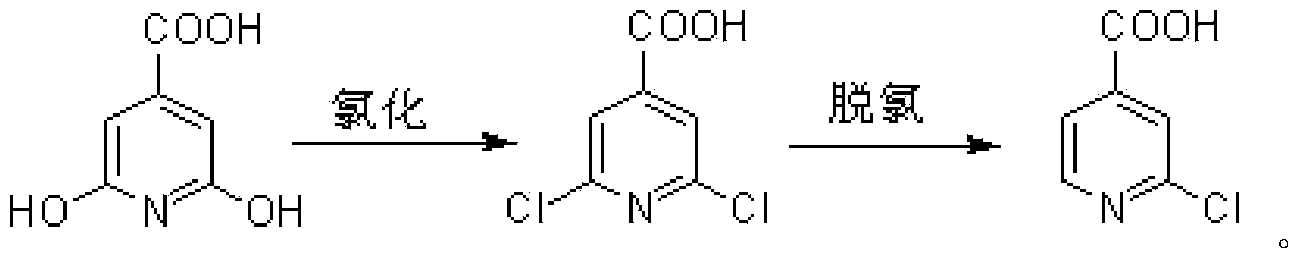
[0033] Embodiment 1: Preparation of 2,6-dichloroisonicotinic acid
[0034] Citric acid (77.6g, 0.5mol), (CH 3 ) 4 NCl (57.5g, 0.525mol) and triphosgene (137mL, 1.5mol) were sequentially added into a 1L four-neck flask; stirred, heated in an oil bath, and reacted at 130°C for 12 hours; after the reaction solution was cooled to room temperature, it was slowly added dropwise to About 1Kg on ice, stirred for 2 hours, then filtered; the resulting filtrate was added with NaHCO 3 Adjust the pH to weak acidity, let stand, separate out unreacted citrazinic acid, suction filter, reclaim to obtain 7.8g citric acid; the solid obtained by filtering in the previous step is dissolved with ethyl acetate after drying, and then filtered to remove insoluble matter, the filtrate After removal of ethyl acetate in vacuo, 78.3 g of 2,6-dichloroisonicotinic acid was obtained as a light yellow solid with a yield of 90.6% (based on the actual consumption of citrazine). m.p.: 209.1~210.7℃.
[0035] ...
Embodiment 2
[0037] Embodiment 2: Preparation of 2-chloroisonicotinic acid
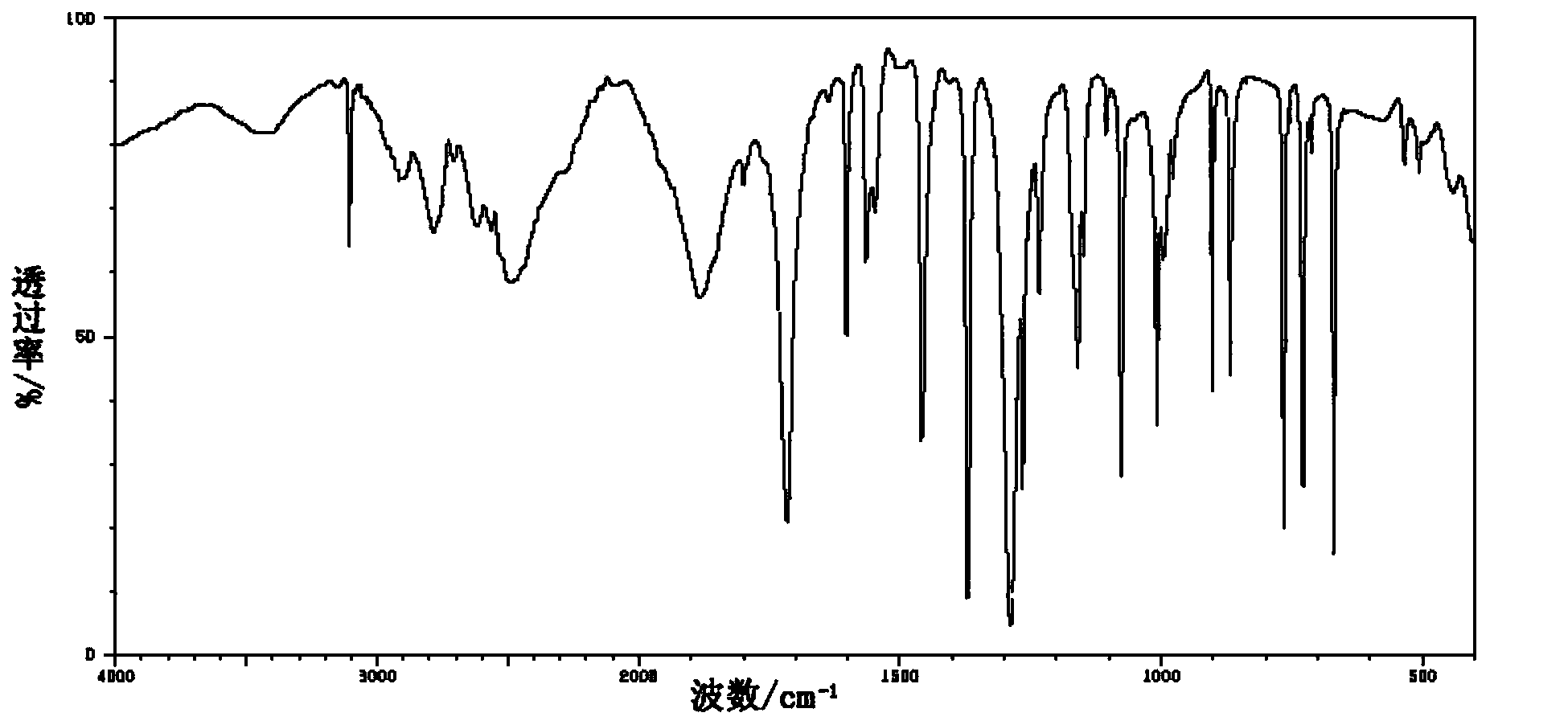
[0038] Put 2,6-dichloroisonicotinic acid (38.4g, 0.2mol) and 80wt% hydrazine hydrate (240g, 3.8mol) into a 2L four-neck flask, stir, heat to 45°C in a water bath, and react for 3 hours; In addition to residual hydrazine hydrate, the resulting brown solid was dissolved with a small amount of water; the resulting solution was heated to boil, and 640 mL of 10 wt% CuSO 4 Solution; continue to boil for 15 minutes, adjust it to strong alkalinity with NaOH solution, and boil for 15 minutes; suction filter while hot, acidify the filtrate with hydrochloric acid to precipitate solids, and obtain 16.3g of 2-chloroisonicotinic acid by suction filtration, which is a white solid. The yield was 51.8%. m.p.: 231.8-233.3°C.
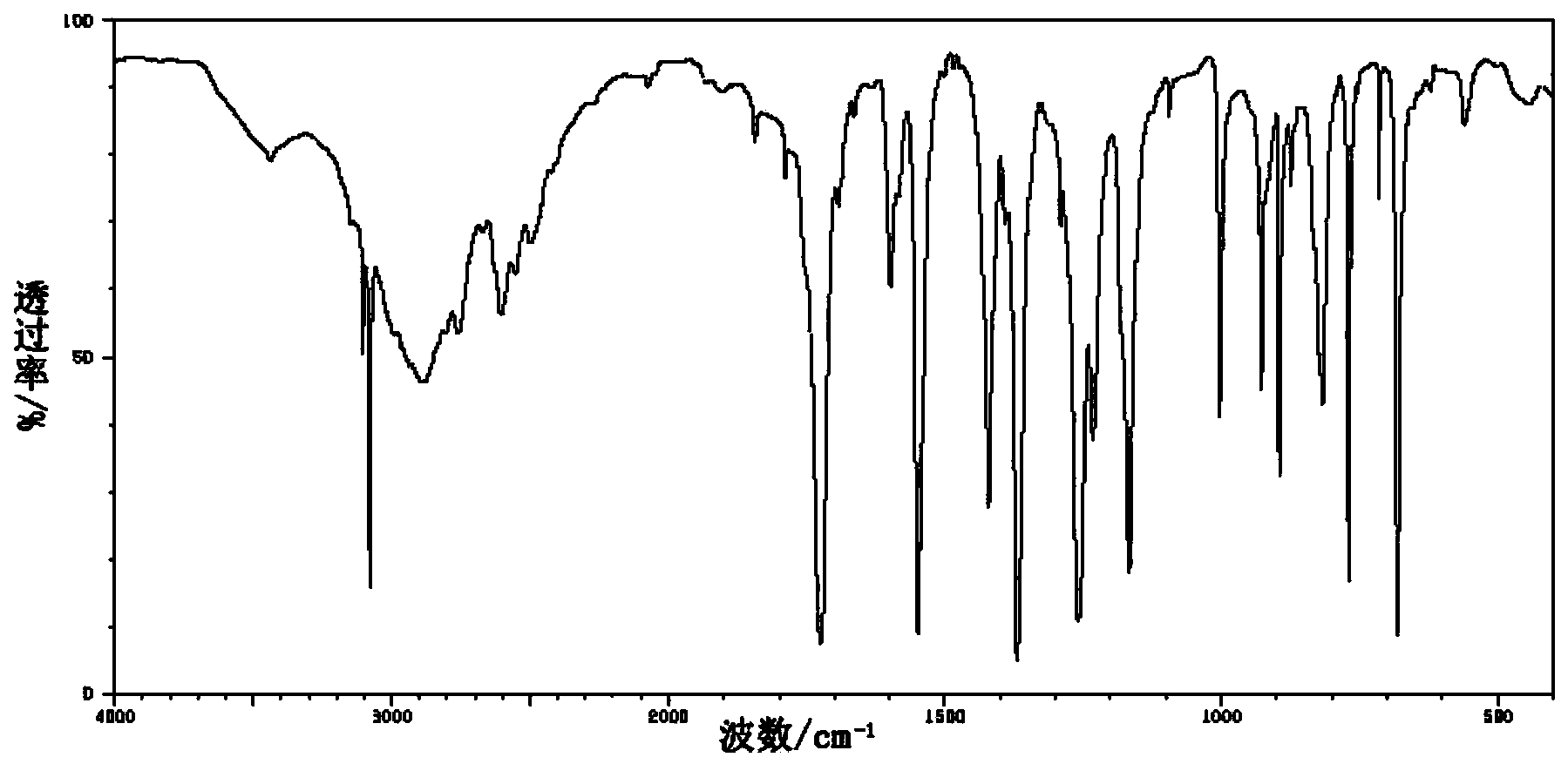
[0039] Spectral data of 2-chloroisonicotinic acid
[0040] IR(KBr,cm -1 ):3106,2917,2904,2784,2706,2617,2662,2494,2484,1884,1800,1716,1603,1566,1548,1459,1266,1234,1161,1160,1077,1008,997,903,868,766,729,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com