Fenbendazole dry suspension and preparation method thereof
A technology of dry fenbendazole and fenbendazole, which is applied in the field of medicine, can solve the problems of low quality stability between batches and inconvenient use, and achieve the effects of good application prospects, convenient portability, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The preparation of embodiment 1 pharmaceutical composition of the present invention
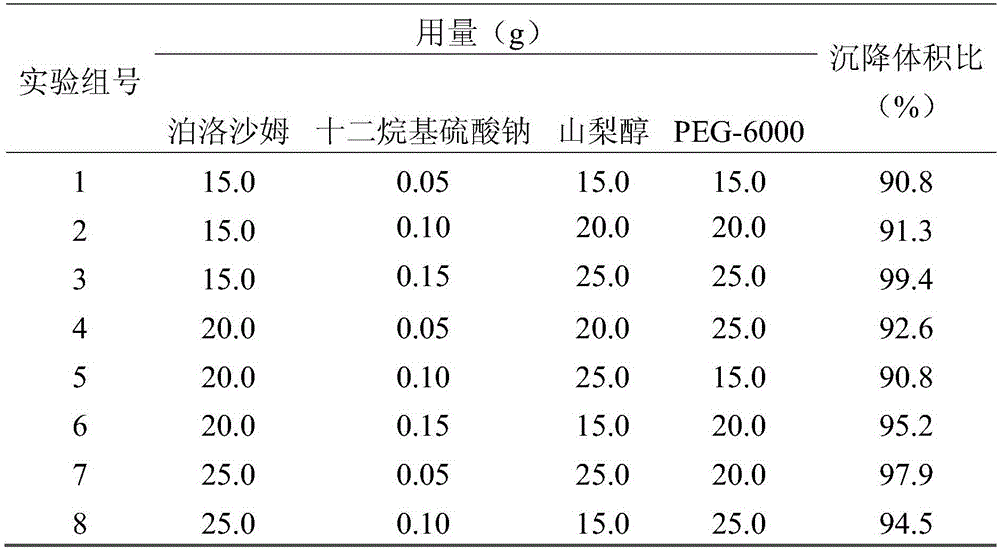
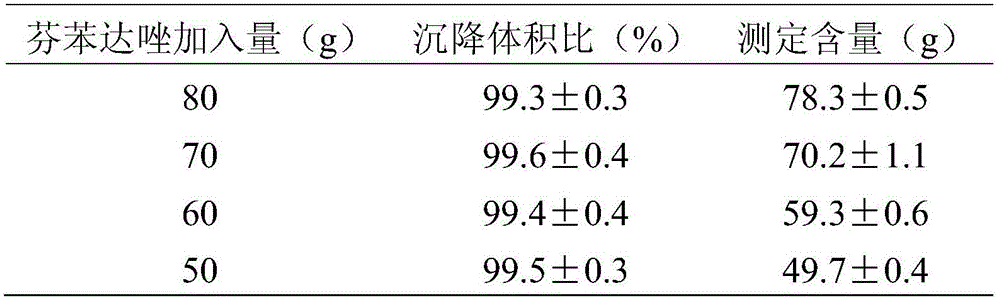
[0019] Prescription: Bendazole 50g, Poloxamer 18825g, Sodium Lauryl Sulfate 0.15g, Sorbitol 20g and PEG-600025g.
[0020] Preparation method: add sorbitol, poloxamer 188, PEG-6000, and sodium lauryl sulfate into water, heat to dissolve and boil, then add fenbendazole while it is hot, and stir in a tank mixer until it becomes granular , taken out, dried, and pulverized until completely passing through a No. 9 sieve (75 μm), to obtain fenbendazole dry suspension.
Embodiment 2
[0021] The preparation of embodiment 2 pharmaceutical composition of the present invention
[0022] Prescription: fenbendazole 70g, poloxamer 18825g, sodium lauryl sulfate 0.15g, sorbitol 20g and PEG-600025g.
[0023] Preparation method: add sorbitol, poloxamer 188, PEG-6000, and sodium lauryl sulfate into water, heat to dissolve and boil, then add fenbendazole while it is hot, and stir in a tank mixer until it becomes granular , taken out, dried, and pulverized until completely passing through a No. 9 sieve (75 μm), to obtain fenbendazole dry suspension.
Embodiment 3
[0024] The preparation of embodiment 3 pharmaceutical composition of the present invention
[0025] Prescription: fenbendazole 70g, poloxamer 18825g, sodium lauryl sulfate 0.15g, sorbitol 20g and PEG-600025g.
[0026] Preparation method: add sorbitol, poloxamer 188, PEG-6000, and sodium lauryl sulfate into water, heat to dissolve and boil, then add fenbendazole while it is hot, and stir in a tank mixer until it becomes granular , taken out, dried, and pulverized until completely passing through a No. 9 sieve (75 μm), to obtain fenbendazole dry suspension.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com