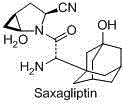
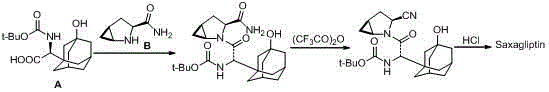
Preparation method of saxagliptin intermediate (A1)
A technology for intermediates and compounds, applied in the field of preparation of saxagliptin intermediates (A1), can solve the problems of many steps and low yields, and achieve the effects of simple operation, stable product quality and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
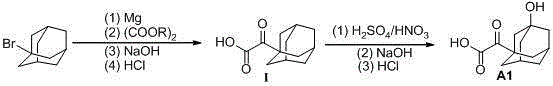
[0045] Example 1: Adamantanone acid ( Ⅰ ) Preparation
[0046] Mg (2.6g, 0.11mol) was added to dry tetrahydrofuran (20mL), under the protection of nitrogen, according to the conventional Grignard reagent preparation, stirred vigorously, heated to 40 ℃, dropwise add bromoadamantane (21.4g, 0.1mol ) Dissolve in dry tetrahydrofuran (100mL), during which a weak reflux is formed. After the dripping is completed, reflux for 1 hour and cool to room temperature to form a Grignard reagent tetrahydrofuran solution.
[0047] In dry tetrahydrofuran (80mL) of diethyl oxalate (29.2g, 0.2mol), cool to -70℃, under nitrogen protection, slowly add the Grignard reagent tetrahydrofuran solution prepared above at this temperature to the solution After the dripping is completed in about 2h, continue to react at this temperature for 4h, slowly add 5mol / L NaOH solution (100mL), while the temperature gradually rises to room temperature, filter to remove insoluble materials, the filtrate continues to stir ...
Embodiment 2
[0048] Example 2: Adamantanone acid ( A1 ) Preparation
[0049] According to the method of introducing hydroxyl groups reported in the patent WO2012028721, after the above product is reacted with mixed acid, the crude product is recrystallized with water to obtain 11.7 g, the yield is 76%, and the melting point is 165-166°C. Purity: 99.2%; 1 HNMR(DMSO-d 6 ): δ=1.49-1.72 (m, 12H), 2.19 (s, 2H), 4.61 (br, 1H), 14.0 (br, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com