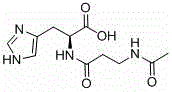
Preparing method for N-acetyl-L-carnosine
A technology of acetyl and carnosine, applied in the field of preparation of N-acetyl-L-carnosine, can solve the problems of complex process, troublesome processing, low yield and the like, and achieve the effects of high HPLC purity, short synthesis route and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of N-acetyl-β-alanine (I)
[0037] In a 250mL reaction flask, add 180mL of chloroform, 30g of β-alanine (0.337mol), 37.8g of acetic anhydride (0.371mol), raise the temperature to 60°C, and react for 5 hours. The sampled ninhydrin does not develop color, and the temperature is lowered to 4°C , stirred for 1 hour, suction filtered to obtain a solid, and dried to obtain 42 g of product (I), the yield was 95.4%, and the HPLC purity was ≥99.3%.
Embodiment 2
[0039] Preparation of N-acetyl-β-alanine (I)
[0040] In a 500mL reaction flask, add 360mL of dichloromethane, 60g of β-alanine (0.674mol), 48.5g of acetic acid (0.808mol), raise the temperature to 45°C, and react for 3 hours. The sampled ninhydrin does not develop color, and the temperature is lowered to 4 ℃, stirred for 1 hour, filtered to obtain a solid, and dried to obtain 82.5 g of product (I), with a yield of 93.4% and an HPLC purity of ≥99.3%.
Embodiment 3
[0042] Preparation of N-acetyl-β-alanine (I)
[0043] In a 1000mL reaction flask, add 360mL toluene, 120g β-alanine (1.348mol), 111.2g acetyl chloride (1.417mol), heat up to 50°C, react for 5 hours, sample ninhydrin does not develop color, cool down to -3 °C, stirred for 1 hour, filtered with suction to obtain a solid, and dried to obtain 169.0 g of product (I), with a yield of 95.7% and an HPLC purity of ≥99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com