Impurity detection method for lansoprazole-containing raw material medicine
The technology of lansoprazole and detection method is applied in the field of determination method of related substances of lansoprazole raw materials and preparations, and can solve the problems such as inability of baseline separation of main component peaks, loss of column bed packing, tailing of chromatographic peaks, and the like, To achieve the effect of simplifying the solution preparation method, reducing the pressure and improving the product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Take lansoprazole for injection as an example to detect impurities.
[0044] Accurately weigh lansoprazole for injection (preparation weighs the content or the appropriate amount of fine powder is approximately equivalent to 50mg), put it in a 50ml brown measuring bottle, add methanol to dissolve and dilute to the mark, shake well (preparation is filtered), and take the subsequent filtrate As the test solution.
[0045] Accurately measure an appropriate amount of test solution, add mobile phase to be mixed with a solution containing 1 μg / mL lansoprazole as a contrast solution.
[0046] Accurately weigh the appropriate amount of impurities A, B, D, and E, add methanol to dissolve and dilute to make a mixed impurity stock solution, and the mass concentrations of impurities A, B, D, and E in the mixed impurity stock solution are 10 μg / mL and 40 μg / mL, respectively , 10 μg / mL, 10 μg / mL mixed impurity stock solution.
[0047] Get another 1ml of mixed impurity stock solution ...
Embodiment 2
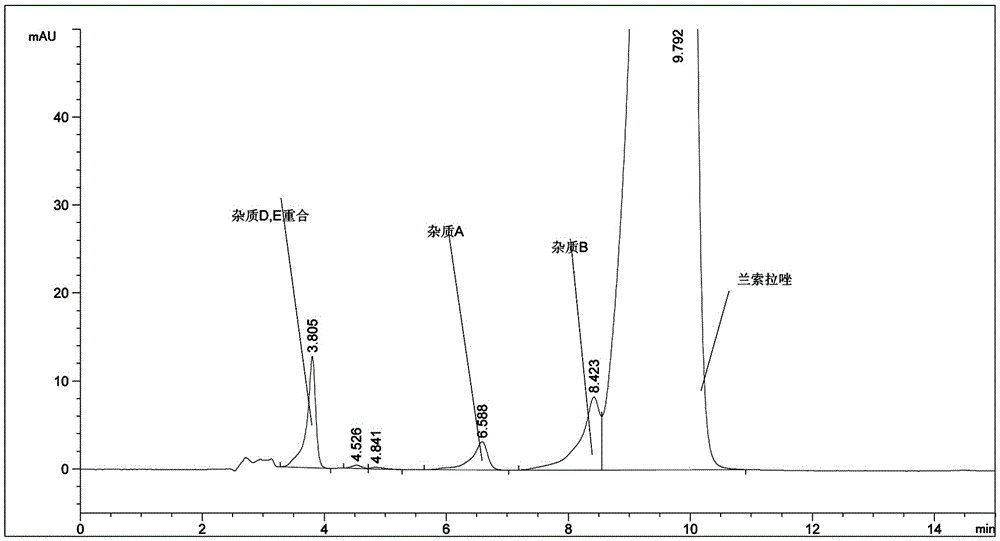
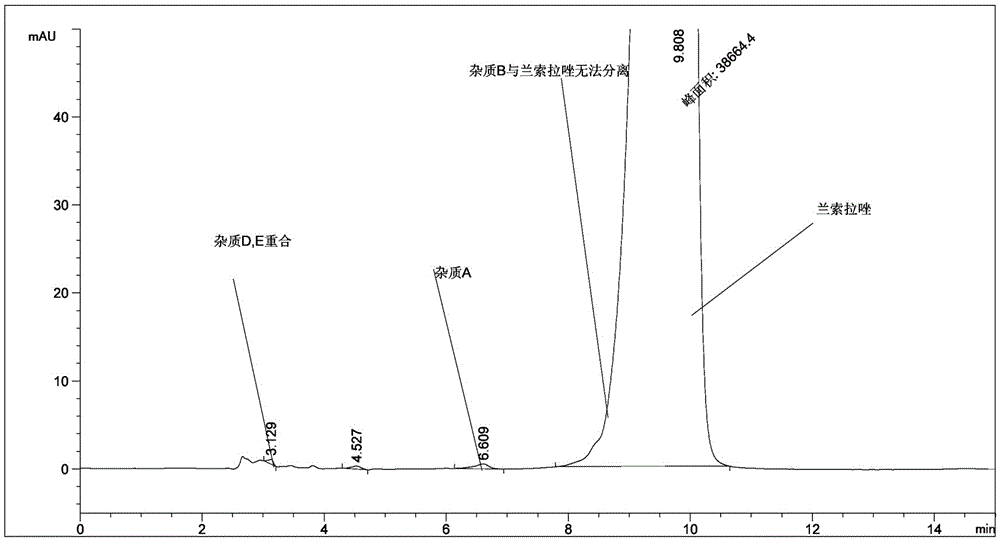
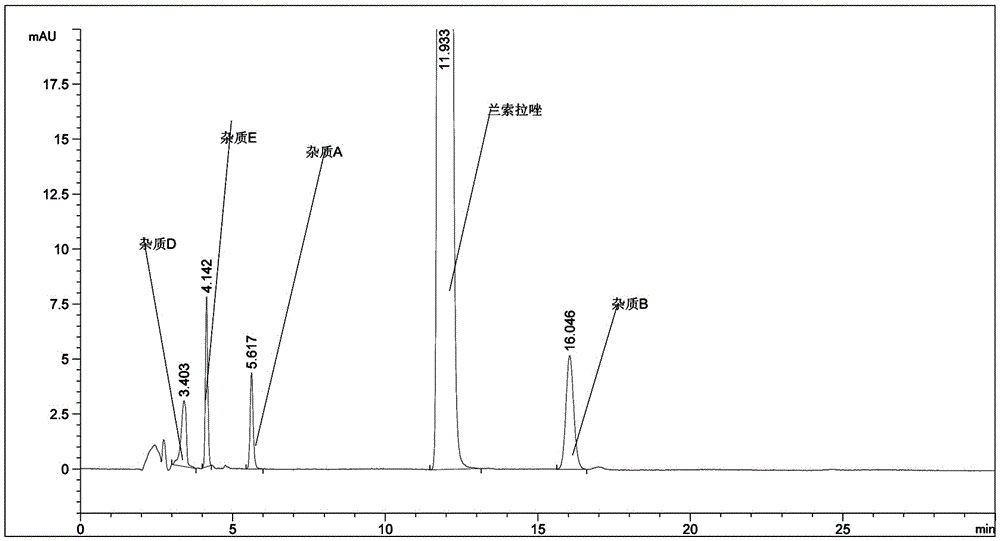
[0056] The raw materials of lansoprazole, lansoprazole for injection, and lansoprazole enteric-coated tablets produced by our company were tested according to the newly developed assay method according to the law. Figure 5-7 .
[0057] Figure 5 Actual picture of related substances of lansoprazole API.
[0058] Figure 6 The real picture of related substances of lansoprazole for injection.
[0059] Figure 7 Lansoprazole Enteric-Coated Tablets Related Substances.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com