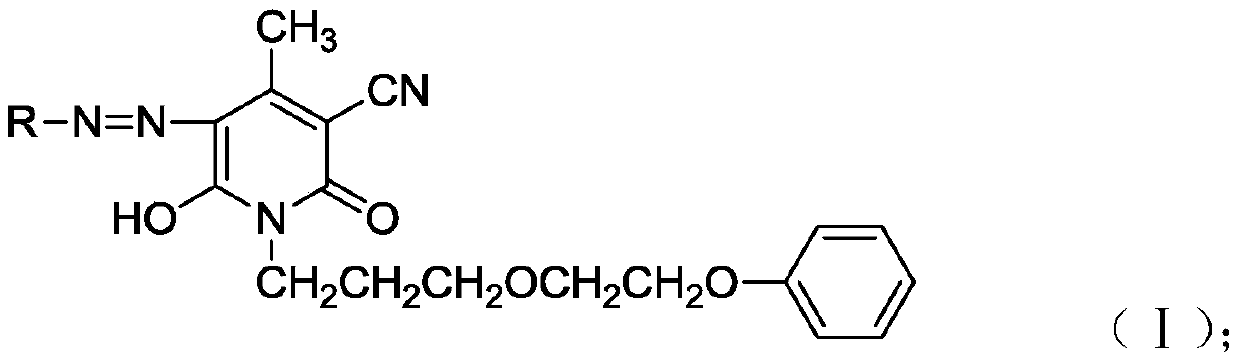
Single azo based pyridine ketone dye and preparation method and application thereof
A technology of monoazopyridone and dyes, applied in monoazo dyes, azo dyes, dyeing methods, etc., can solve the problems of poor thermal migration fastness and sublimation fastness, and achieve dry cleaning and sun resistance Effects of fastness, color stability, affinity enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
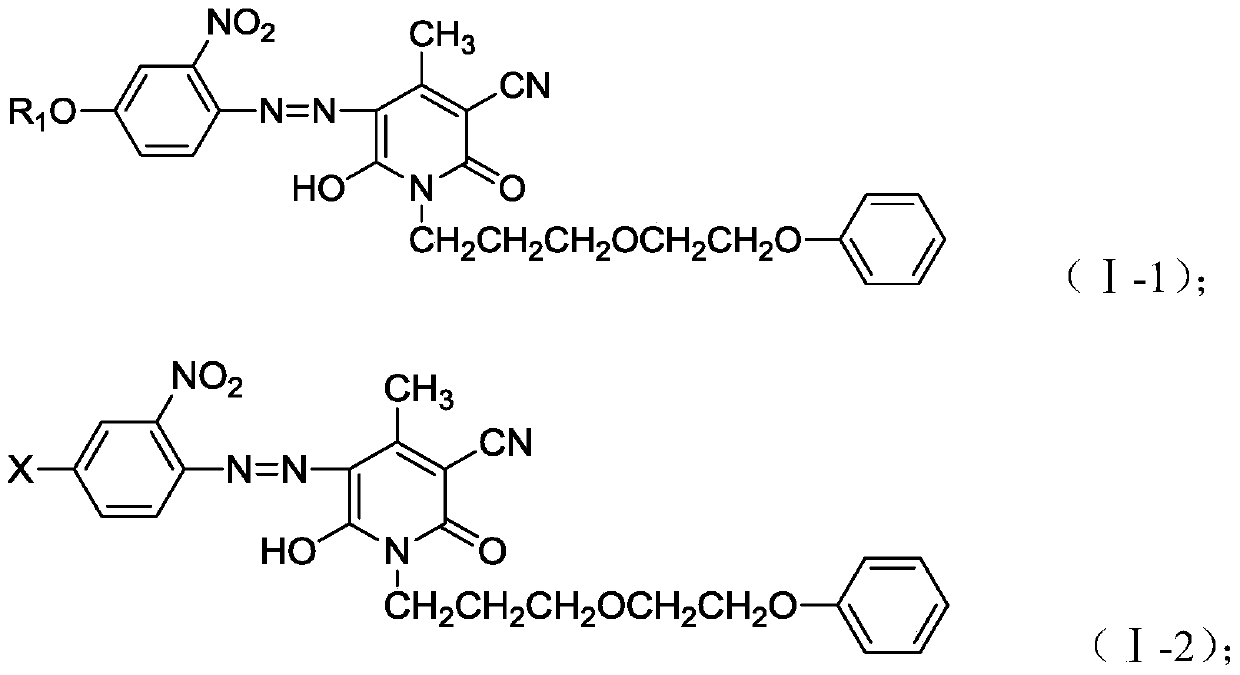
[0035] In this embodiment, a monoazopyridone dye has a structural formula as shown in formula (I-1):
[0036]
[0037] The preparation method of this monoazo pyridone dye is:
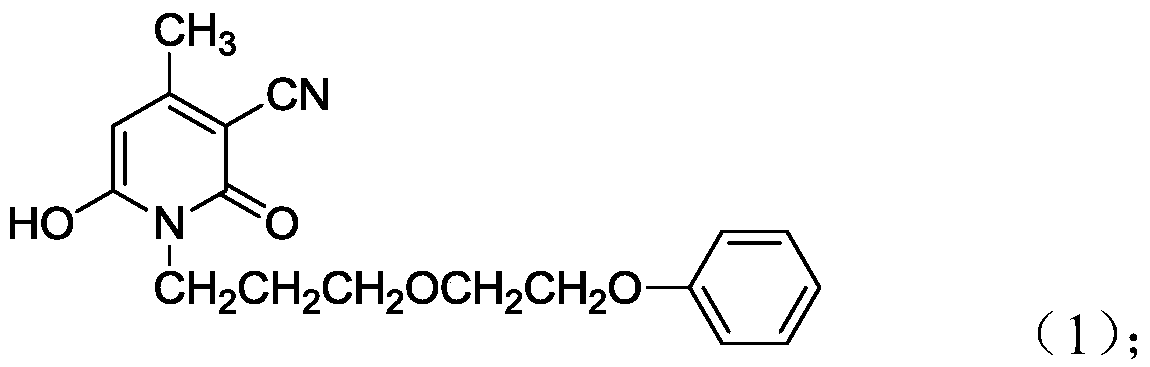
[0038] 1) Mix water, methyl cyanoacetate and phenoxyethoxypropylamine at a volume ratio of 1:1:2 at 50-55°C for 2 hours, then add methyl acetoacetate and raise the temperature to 85-90°C for reaction In 12 hours, the coupling component shown in formula (1) was obtained:
[0039]
[0040] 2) Add 33g of nitrosyl sulfuric acid into a 100ml flask, control the temperature at 10-30°C, slowly add 16.8g of 2-nitro-4methoxyaniline into the flask, and keep warm until the diazo complete (a small amount to clear and transparent in ice water is qualified), and obtain the diazonium salt;
[0041] 3) Add 400mL of water, 80mL of sodium acetate, and 60mL of coupling components to a 1000ml beaker, stir until completely dissolved, add ice to control the temperature at about 0°C, slowly add the prepared diazonium s...
Embodiment 2
[0043] In this embodiment, a monoazopyridone dye has a structural formula as shown in formula (I-2):
[0044]
[0045] The preparation method of the monoazopyridone dye refers to Example 1.
Embodiment 3
[0047] A monoazo pyridone dye in this embodiment has a structural formula as shown in formula (I-3):
[0048]
[0049] The preparation method of the monoazopyridone dye refers to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com