Method for detection effectiveness of aftosa inactivated vaccines
A technology of inactivated vaccines and testing methods, applied in the field of biological products for animals, can solve problems such as outbreaks of epidemics, large individual differences in animals, and inability to objectively reflect the effective antigen content of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
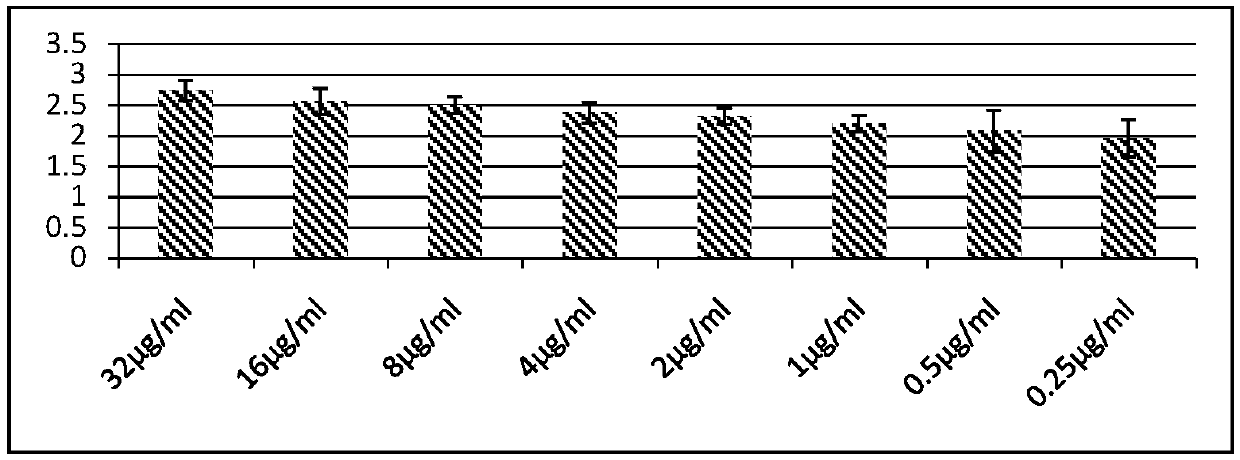
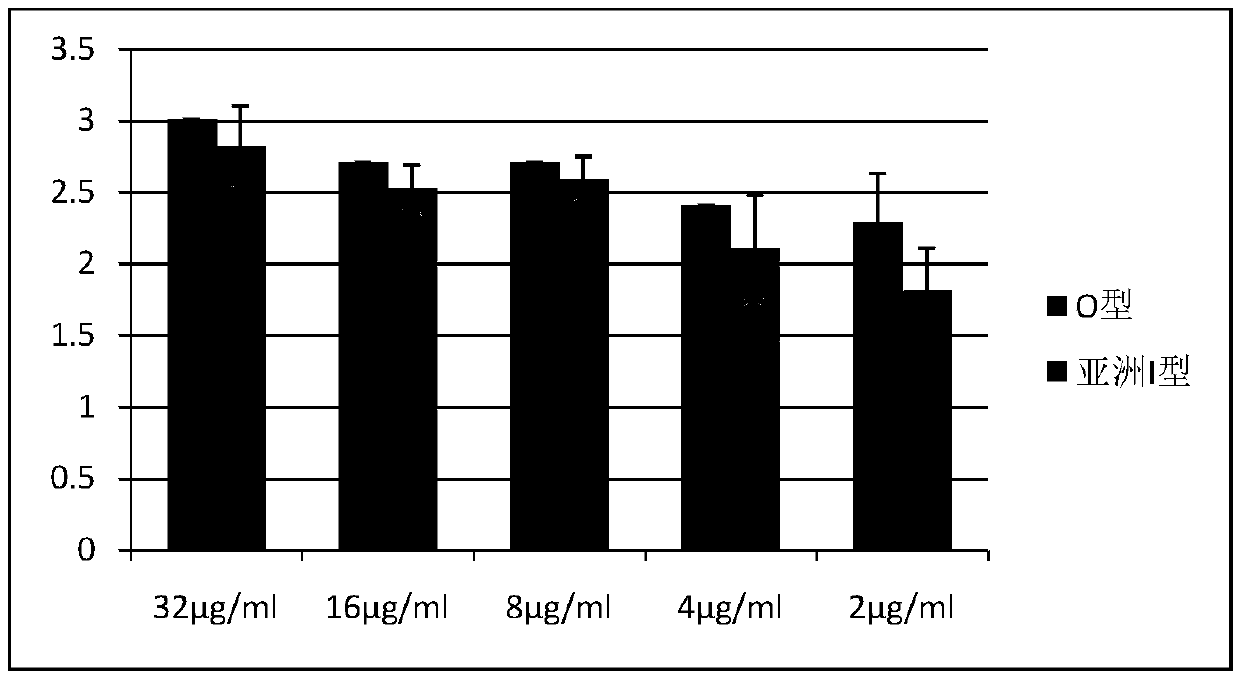
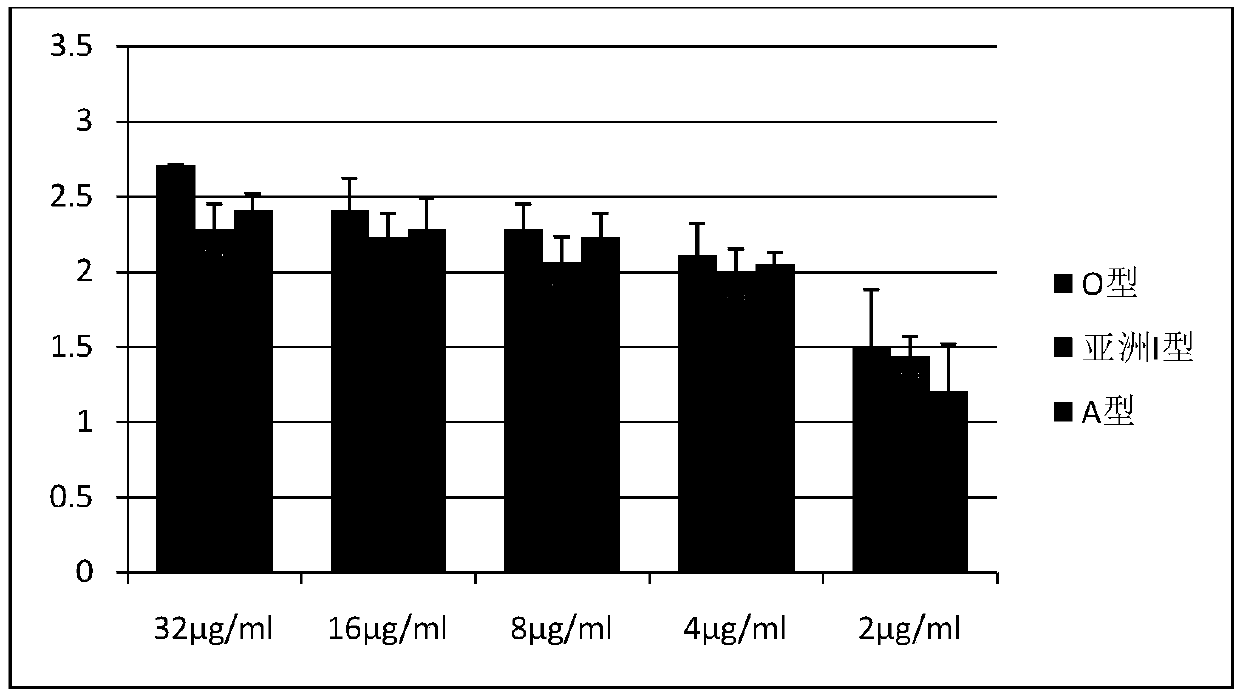
[0013] 1. The relationship between different 146S content of foot-and-mouth disease O-type inactivated antigen and mouse immune serum antibody
[0014] The O-type foot-and-mouth disease inactivated antigen (provided by Jinyu Baoling Biological Pharmaceutical Co., Ltd.) with known 146S content (64 μg / ml) was serially diluted to 0.25 μg / ml, and an equal volume of 206 adjuvant (China Animal Husbandry Co., Ltd. Provided by Lanzhou Biopharmaceutical Factory) prepared vaccine (vaccine antigen content--32μg / ml), each Balb / c was subcutaneously injected with 0.5ml for immunization, each antigen content vaccine was injected into 5 mice, blood was collected for 28 days to separate serum, and liquid phase Serum titer was determined by ELISA kit. The serum titer is represented by -log10X, and the mean value and standard deviation are calculated, and the results are as follows figure 1 shown.
[0015] The antigen content per milliliter of vaccine is correlated with antibody production, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com