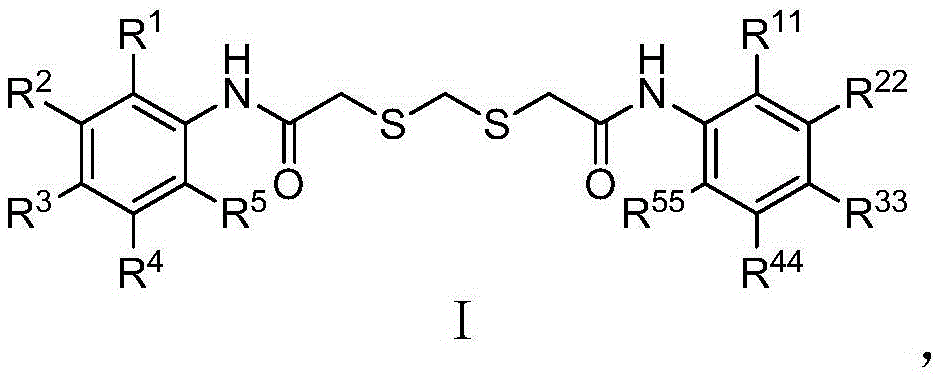
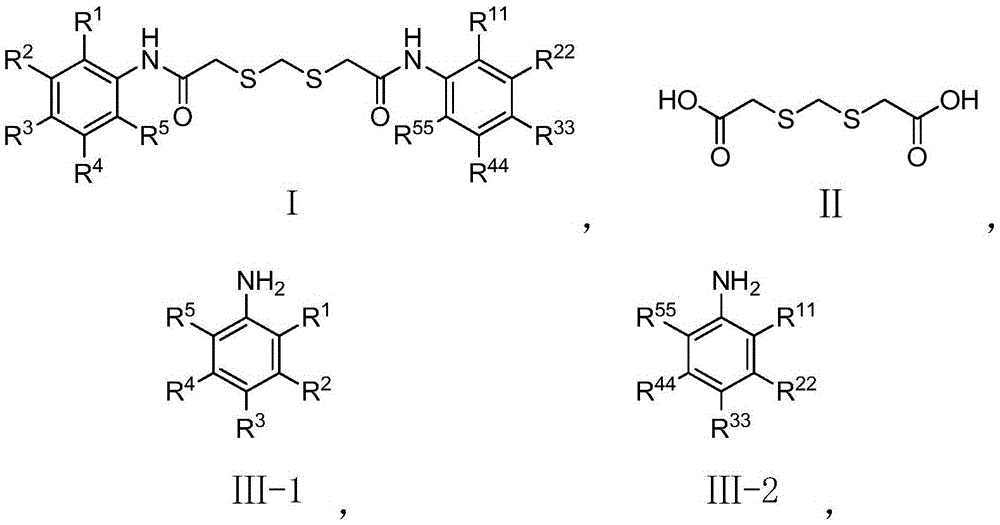
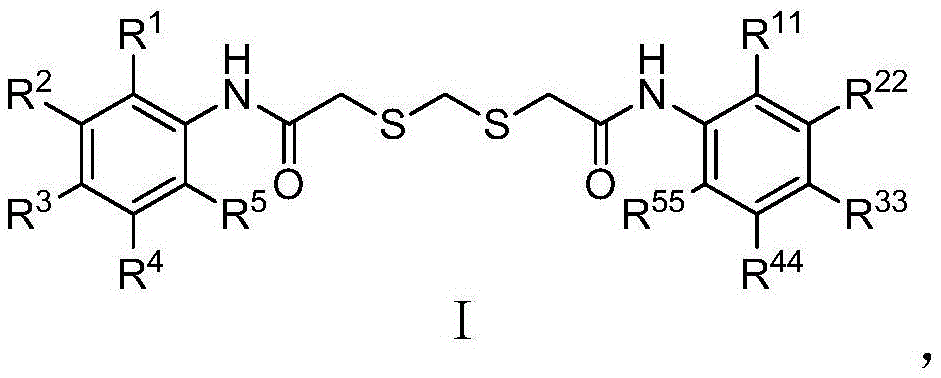
Compound inhibiting algae growth and preparation method thereof and algicide and application of compound and algicide
A compound, algicide technology, applied in herbicides and algicides, botanical equipment and methods, biocides, etc., can solve the problems of cyanobacteria cannot grow normally, die and other problems, achieve low toxicity, high inhibition rate, Highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0070] Preparation of N,N'-bis(4-chlorophenyl)methylenebisthioacetamide:
[0071]
[0072] The first step: mix paraformaldehyde (20mmol) solution (40% by weight solution) with mercaptoacetic acid (40mmol) solution (90% by weight solution), add 0.2mL concentrated hydrochloric acid dropwise to the mixed solution under stirring, Stir overnight at 45°C, then stir at 95°C for 2 hours, then store at minus 18°C for 48 hours, filter the resulting mixture with suction, and recrystallize with water to obtain pure intermediate II with a yield of 60%;
[0073] Step 2: Add 3mmol of compound II and 6.6mmol of p-chloroaniline to 6.0mL of anhydrous DMF, protect with Ar, cool to 0°C in an ice-water bath, and then dissolve 6.6mmol of DCC in 2mL of anhydrous DMF , and then add the DCC solution dropwise to the above mixture, and control the addition for 3 minutes. Stir at room temperature for 2 hours, stand overnight, filter, pour the filtrate into 80mL water, precipitate white precipitate,...
preparation example 2
[0078] Preparation of N,N'-bis(4-iodophenyl)methylenebisthioacetamide:
[0079]
[0080] It was prepared by a method similar to that of Preparation Example 1, except that p-iodoaniline was used instead of p-chloroaniline in Preparation Example 1.
[0081] The obtained pure product is a white powder with a yield of 22%, m.p.241-242°C.
[0082] Molecular formula: C 17 h 16 I 2 N 2 o 2 S 2 ;
[0083] 1 HNMR (600MHz, DMSO-d 6 ) δ 10.24 (s, 2H), 7.64 (d, J = 8.5Hz, 4H), 7.42 (d, J = 8.5Hz, 4H), 4.01 (s, 2H), 3.45 (s, 4H).
[0084] 13 CNMR (151MHz, DMSO-d 6 ) δ 167.76, 138.77, 137.41, 121.38, 86.98, 35.90, 34.64.
preparation example 3
[0086] Preparation of N,N'-bis(4-bromophenyl)methylenebisthioacetamide:
[0087]
[0088] It was prepared by a method similar to Preparation Example 1, except that p-bromoaniline was used instead of p-chloroaniline in Preparation Example 1.
[0089] The obtained pure product is a white powder with a yield of 52%, m.p.214-216°C.
[0090] Molecular formula: C 17 h 16 Br 2 N 2 o 2 S 2 ;
[0091] 1 HNMR (600MHz, DMSO-d 6 ) δ 10.28 (s, 2H), 7.55 (d, J = 8.7Hz, 4H), 7.48 (d, J = 8.6Hz, 4H), 4.01 (s, 2H), 3.46 (s, 4H).
[0092] 13 CNMR (151MHz, DMSO-d 6 ) δ 168.18, 138.73, 132.00, 121.53, 115.43, 36.32, 35.03.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com