Paclitaxel (PTX) derivative, its preparation method and its use
A technology of paclitaxel derivatives and surfactants, which is applied in pharmaceutical formulations, drug combinations, and medical preparations containing active ingredients, etc., can solve problems such as acute allergic reactions, low drug concentrations, and reduced concentrations, so as to avoid allergic reactions, The effect of multidrug resistance inhibition and drug concentration enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8
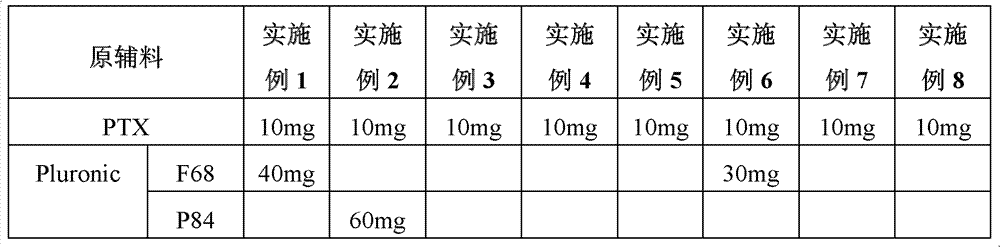
[0016] The preparation method is: according to the dosage shown in Table 1, mix different types of Pluronic and 3-3'-dithiodipropionic acid, add dimethylaminopyridine and 1-ethyl-(3-dimethylaminopropyl ) carbodiimide hydrochloride, dissolved in dichloromethane, kept in a water bath at 25°C for 48 hours, added PTX and kept warm for 24 hours, after vacuum drying, the sample was dissolved in absolute ethanol, added water for injection and ultrasonically placed in Dialyze in a dialysis bag of 3500 Daltons to obtain paclitaxel derivatives of the present invention (ie substances with a molecular weight greater than 3500 Daltons).
[0017] Table 1 prepares the raw material consumption of various paclitaxel derivatives
[0018]
[0019]
Embodiment 1
[0021] Paclitaxel derivatives prepared in Example 1: δ1.17ppm is CH in Pluronic 2 CH(CH 3 ) The methyl characteristic peak of O, δ3.3-3.7ppm is CH in Pluronic 2 CH(CH 3 )O and CH 2 CH 2 The characteristic peak of the methylene group of O; δ7.2-7.8ppm is the characteristic peak of the benzene ring in paclitaxel, and δ2.2ppm is the -OOC-CH in paclitaxel 3 The characteristic peak of methyl group; δ2.4ppm, δ2.8ppm belongs to -OOCCH 2 CH 2 SSCH 2 CH 2 The characteristic peak of methylene in COO-. From 1 There are both paclitaxel and Pluronic peaks and disulfide bond peaks on H-NMR, which proves the formation of paclitaxel derivatives.
Embodiment 2
[0022] Paclitaxel derivatives prepared in Example 2: δ1.18ppm is CH in Pluronic 2 CH(CH 3 ) The methyl characteristic peak of O, δ3.3-3.7ppm is CH in Pluronic 2 CH(CH 3 )O and CH 2 CH 2 The characteristic peak of the methylene group of O; δ7.2-7.8ppm is the characteristic peak of the benzene ring in paclitaxel, and δ2.2ppm is the -OOC-CH in paclitaxel 3 The characteristic peak of methyl group; δ2.4ppm, δ2.8ppm belongs to -OOCCH 2 CH 2 SSCH 2 CH 2 The characteristic peak of methylene in COO-.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com