Preparation method of 2,6-pyridinedimethanol
A technology of pyridinedimethanol and lutidine, applied in 2 fields, can solve problems such as corrosiveness, increased difficulty of reaction, cumbersome operation steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
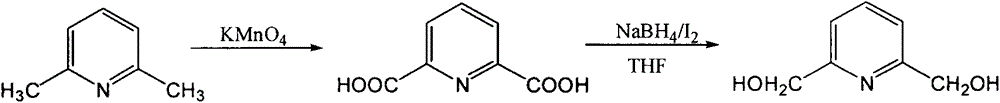
[0009] (1) Synthesis of 2,6-pyridinedicarboxylic acid:
[0010] In a 2L flask equipped with a stirrer and a reflux condenser, add 800mL of water, 53.5g of KMnO 4 , 16.7g2,6-lutidine, heated to reflux, when KMnO 4 When the color fades, add another 53.5gKMnO 4 And 200mL water, continue to reflux until the color fades completely (about 2h), cool, filter out MnO 2 , the filtered insoluble matter was washed in 500 mL of hot water, filtered, and the filtrate was combined, the filtrate was concentrated to 200-300 mL, filtered, then acidified with concentrated HCl, cooled, a precipitate was precipitated, filtered, and dried to obtain 2,6 - picolinic acid. Yield: 80%, m.p. 220°C, consistent with literature.
[0011] (2) Synthesis of 2,6-pyridinedimethanol:
[0012] Add 8.8 g (0.05 mol) of 2,6-pyridinedicarboxylic acid and 200 mL THF into a 500 mL three-necked flask, cool to -5 degrees Celsius in an ice-salt bath, and add 3.8 g (0.1 mol) NaBH in batches while stirring. 4 . React ...
Embodiment 2
[0014] (1) Synthesis of 2,6-pyridinedicarboxylic acid:
[0015] In a 500 four-neck flask equipped with a stirrer and a reflux condenser, add 300 mL of water and 10.7 g (0.1 mol) of 2,6-lutidine, and heat to 60 degrees. 0.5molKMnO 4 Add in small amounts in batches, control the temperature at 85-90 degrees, and continue the reaction until the color fades completely (about 2h). Cool, filter out MnO 2 , wash with 100mL of hot water, filter, combine the filtrates, concentrate to 100-130mL, and then acidify with concentrated HCl, cool to precipitate, filter, and dry to obtain 2,6-pyridinedicarboxylic acid. Yield: 78%, m.p. 220°C, consistent with literature.
[0016] 2) Synthesis of 2,6-pyridinedimethanol:
[0017] Add 8.8g (0.05mol) of 2,6-pyridinedicarboxylic acid and 200mLTHF into a 500mL three-necked flask, and add 7.6g (0.2mol) of NaBH in batches under stirring at room temperature 4 . React for half an hour after the addition, until no more gas is generated. Dissolve 0.05...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com