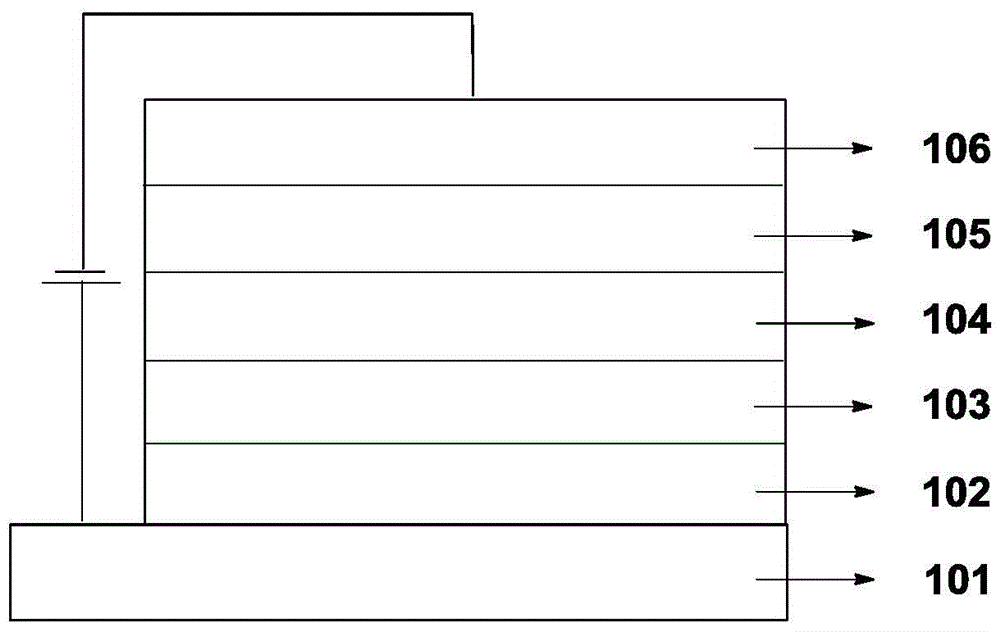
Small molecular organic electroluminescent material and application thereof
A technology of electroluminescent materials and small molecules, which is applied in the fields of luminescent materials, organic chemistry, circuits, etc., can solve the problems of fluorescent wavelength, poor optical data of luminous efficiency, lack of rigidity of molecules, etc., and achieve excellent color purity and device efficiency Excellent, good film stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 compound 1
[0048]
[0049] In a 2L three-necked flask, add 1,8-dibromonaphthalene (81.2g, 0.28mol), 1-naphthaleneboronic acid (53.6g, 0.31mol), potassium carbonate (78.4g, 0.57mol), toluene (540g), and Ionized water (200g), absolute ethanol (180g), under nitrogen protection, add Pd(PPh 3 ) 4 (1.1g), heat up to reflux, heat preservation reaction for 10h, cool down to 25°C, separate liquid, wash the organic phase once with 200g deionized water, dry with 100g anhydrous sodium sulfate, filter with suction, collect the organic phase, and pass the organic phase quickly through a 35cm thick The silica gel column was passed through the column solution to remove the solvent, and the obtained crude product was recrystallized using absolute ethanol as a solvent to obtain compound 1, 65 g of light yellow solid, yield 68.7%, MS (m / s): 333.2.
Embodiment 2
[0050] The preparation of embodiment 2 compound C01
[0051]
[0052] Preparation of compound 2: In a 100mL three-necked flask, add compound 1 (3.3g, 0.01mol), dry tetrahydrofuran (35g), cool down to -78°C, drop n-butyllithium in n-hexane solution (4.5mL, 2.2 mol / L, 0.01mol), kept at -78°C for 1h, dissolved dry bis(4-methylphenyl)methanone (2.1g, 0.01mol) in 15mL of dry tetrahydrofuran, and then slowly dropped it into the reaction flask, Incubate at -78°C for 2.5h, move the reaction bottle into a water bath at 25°C, naturally heat up the reaction solution to 10°C, add 15mL of 10% hydrochloric acid aqueous solution, stir for 5min, separate the liquids, collect the organic phase, remove the solvent, 4.5 g of the crude product of compound 2 was obtained, and the crude product of compound 2 was not further refined, and was directly used in the next reaction.
[0053] Preparation of compound C01: In a 100mL three-necked flask, add the crude product of compound 2 (4.5g) obtained...
Embodiment 3
[0055] The preparation of embodiment 3 compound C05
[0056]
[0057] Using bis(4-tert-butylphenyl)methanone instead of bis(4-methylphenyl)methanone as a raw material, compound C05 was prepared according to the method described in Example 2 to obtain 3.3 g of a white solid with a yield of 62.7%.
[0058] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 41 h 38 , the theoretical value is 530.2974, and the test value is 530.2979. Elemental analysis (C 41 h 38 ), theoretical value C: 92.78, H: 7.22, measured value C: 92.79, H: 7.21.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com