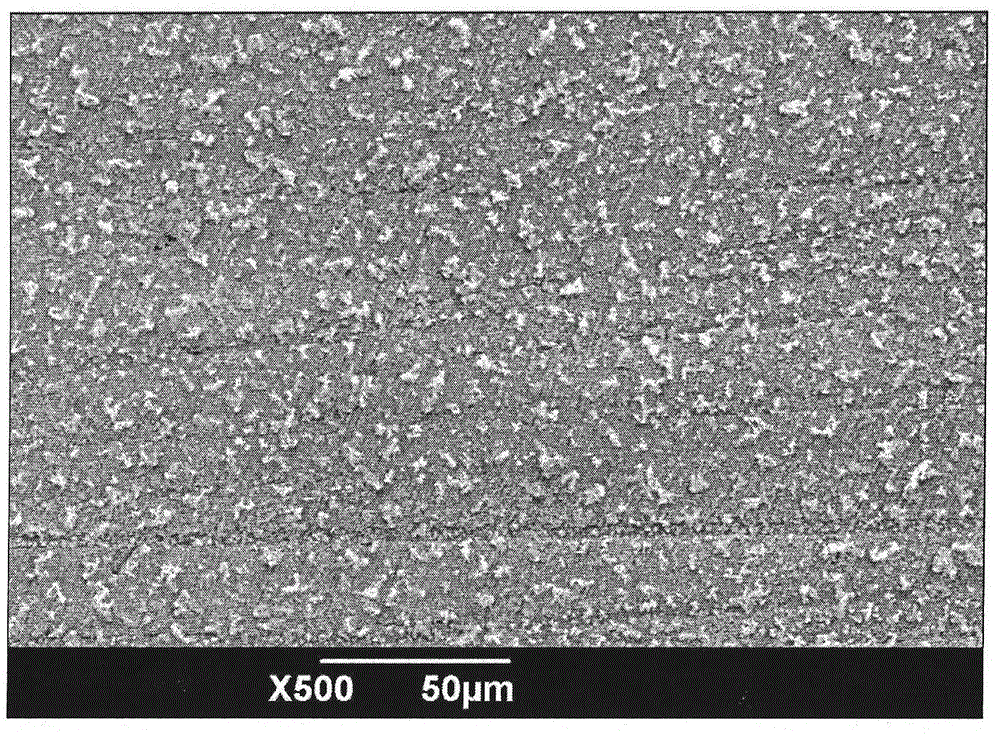
Replacement reaction method for preparation of silver dendritic super-hydrophobic surface
A super-hydrophobic surface and displacement reaction technology, applied in liquid chemical plating, metal material coating process, coating, etc., can solve problems that hinder large-scale application, complex process control, harsh preparation conditions, etc., and achieve super-hydrophobic performance The effect of maintaining stability, widening the concentration range, and shortening the preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Step 1. Dissolve 0.05 g of silver nitrate crystals in distilled water, set the volume to 100 mL, and stir evenly to obtain solution A with a silver nitrate concentration of 0.003 mol / L;
[0026] Step 2. Dissolve 2.28 g of myristic acid in absolute ethanol, set the volume to 100 mL, and stir evenly to obtain a solution B with a concentration of myristic acid of 0.1 mol / L;
[0027] Step 3: Polish the copper substrate with a size of 50mm×25mm×1.5mm with water sandpaper to remove the oxide layer on the surface of the copper substrate, then rinse the polished copper substrate with distilled water and absolute ethanol in turn, and dry it for use;
[0028] Step 4, put the dried copper matrix in step 3 into the solution A described in step 1 to cause a displacement reaction, the time is 50s;
[0029] Step 5. Rinse and dry the copper substrate obtained in step 4 with distilled water and absolute ethanol successively, then apply solution B evenly on the surface of the rinsed and ...
Embodiment 2
[0032] Step 1. Dissolve 0.5 g of silver nitrate crystals in distilled water, set the volume to 100 mL, and stir evenly to obtain solution A with a silver nitrate concentration of 0.0294 mol / L;
[0033] Step 2. Dissolve 1.14 g of myristic acid in absolute ethanol, set the volume to 100 mL, and stir evenly to obtain a solution B with a concentration of myristic acid of 0.05 mol / L;
[0034] Step 3: Polish the copper substrate with a size of 50mm×25mm×1.5mm with water sandpaper to remove the oxide layer on the surface of the copper substrate, then rinse the polished copper substrate with distilled water and absolute ethanol in turn, and dry it for use;
[0035] Step 4, put the dried copper matrix in step 3 into the solution A described in step 1 to cause a displacement reaction, the time is 30s;
[0036] Step 5. Rinse the copper substrate obtained in step 4 with distilled water and absolute ethanol successively, then apply the solution B described in step 2 evenly on the surface o...
Embodiment 3
[0038] Step 1. Dissolve 0.85 g of silver nitrate crystals in distilled water, set the volume to 100 mL, and stir evenly to obtain a solution A with a silver nitrate concentration of 0.050 mol / L;
[0039] Step 2. Dissolve 2.28 g of myristic acid in absolute ethanol, set the volume to 100 mL, and stir evenly to obtain a solution B with a concentration of myristic acid of 0.10 mol / L;
[0040] Step 3: Polish the copper substrate with a size of 50mm×25mm×1.5mm with water sandpaper, 以 Remove the oxide layer on the surface of the copper substrate, then rinse the polished copper substrate with distilled water and absolute ethanol in turn, and dry it for use;
[0041] Step 4, put the dried copper substrate in step 3 into the solution A described in step 1 to cause a displacement reaction, the time is 5s;
[0042] Step 5. Rinse the copper substrate obtained in step 4 with distilled water and absolute ethanol successively, then apply the solution B in step 2 evenly on the surface of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com