Three-cistron expression vector, preparation method and application
An expression vector, tricistron technology, applied in the field of genetic engineering, can solve the problems affecting the quality of monoclonal antibodies, the problems of industrialized production of genetic engineering, positive clones not expressing the target protein, etc., so as to improve the expression level of antibody protein and improve the Clonal selection rate, the effect of improving effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The construction of tricistronic expression vector comprises the following steps:
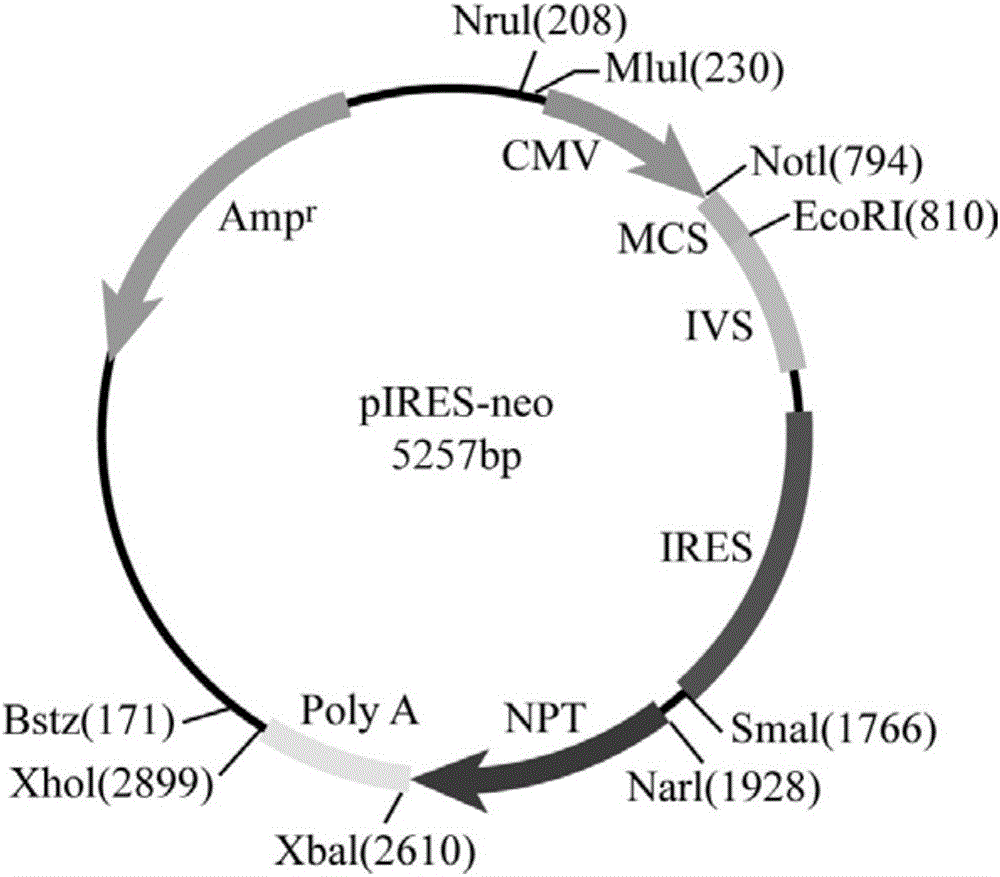
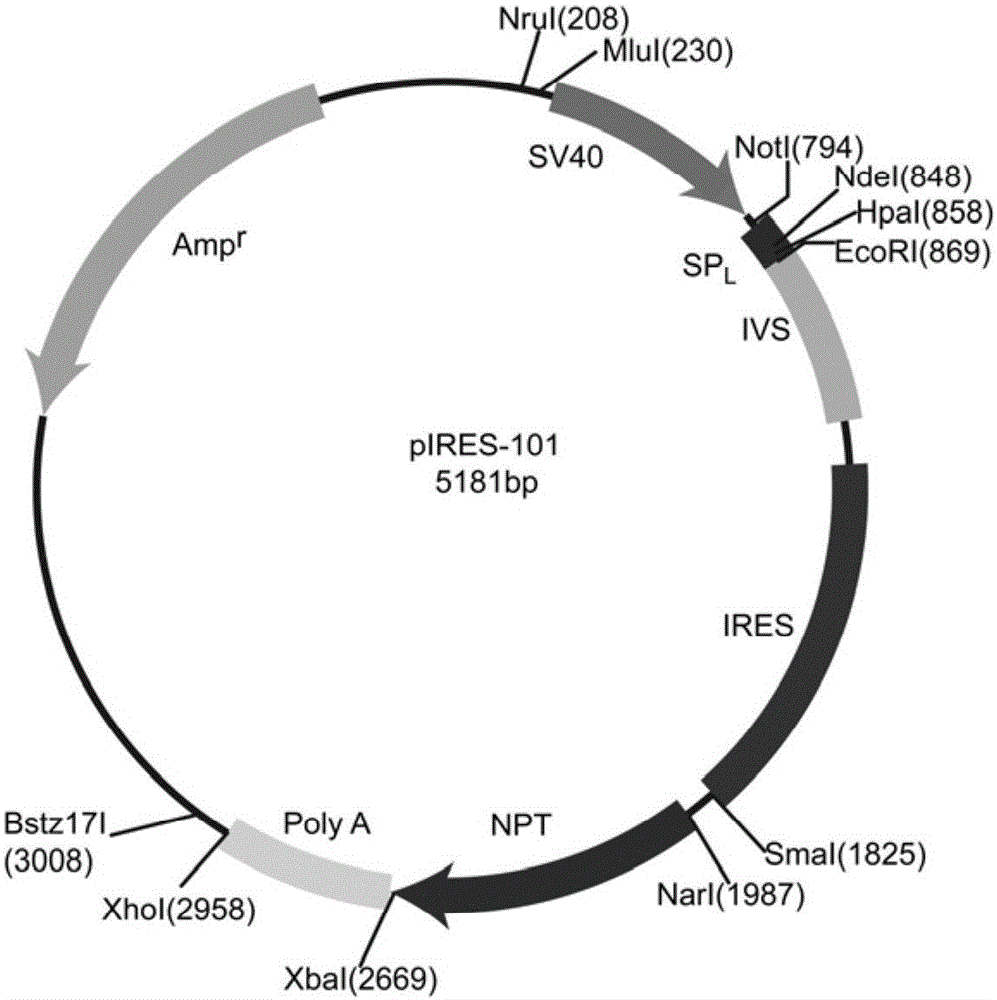
[0039] 1. Construction of pIRES-101 vector (i.e., on the basis of pIRES-Neo vector, replace CMV promoter with SV40 promoter, insert light chain signal peptide sequence, and NdeI, HpaI restriction sites in MCS at the same time)
[0040] 1) Synthesize the fusion sequence of SV40 promoter and light chain signal peptide
[0041] According to the reported SV40 promoter sequence (GenBank accession number: KM359772.1, bases 2794-3247, as shown in SEQ ID NO: 3) and light chain signal peptide sequence (GenBank accession number: Z69026.1, bases 1-66 base, as shown in SEQ ID NO: 4) to artificially synthesize the fusion sequence of SV40 promoter and light chain signal peptide (as shown in SEQ ID NO: 9), which was specifically synthesized by General Biogene (Anhui) Co., Ltd. To facilitate cloning, when synthesizing the fusion sequence, AGCACGCGT sequence was introduced at the 5' end, where AGC was t...
Embodiment 2
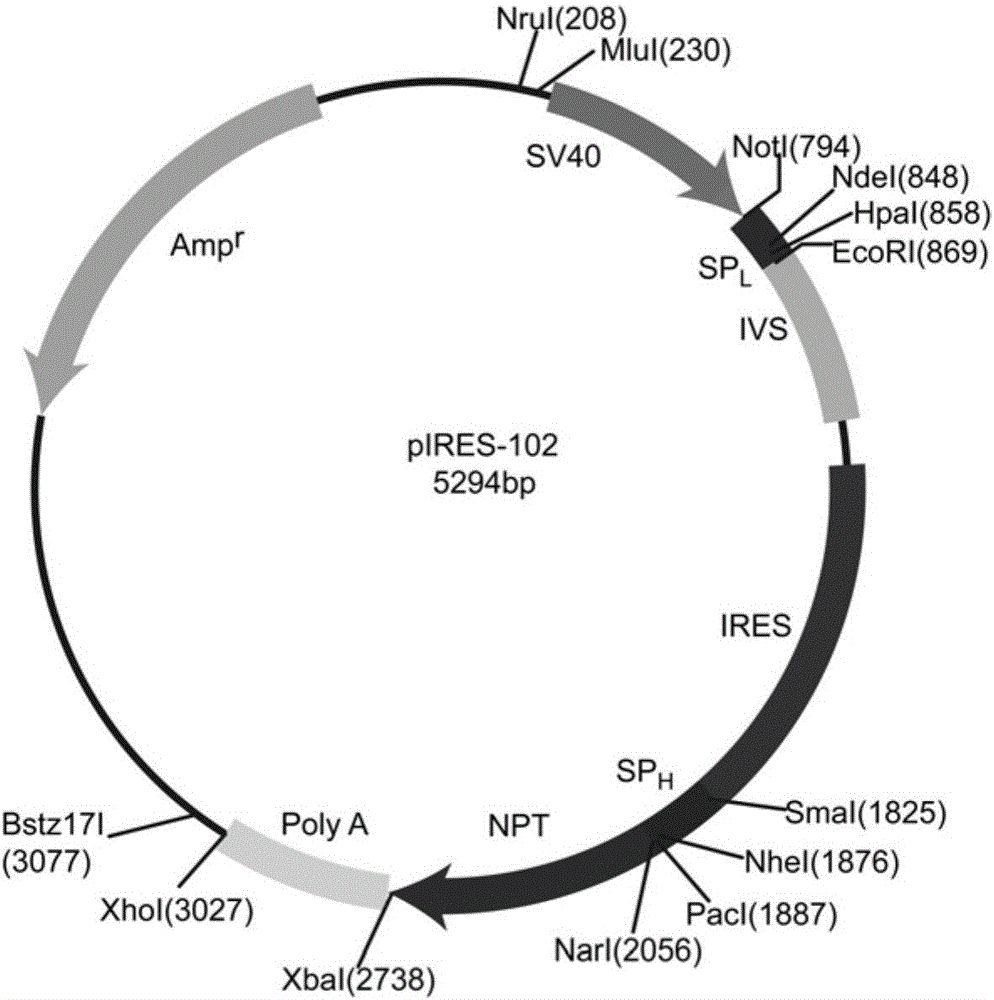
[0092] The construction of the pIRES-106 vector comprises the following steps:
[0093] 1) Artificially synthesized anti-CD20 antibody kappa chain cDNA sequence and constructed pIRES-105C expression vector
[0094] The anti-CD20 antibody kappa chain cDNA sequence (as shown in SEQ ID NO: 14) was artificially synthesized according to the sequence shown in SEQ ID NO: 8, and was synthesized by General Biogene (Anhui) Co., Ltd. For the convenience of cloning, ATACATATG was introduced at the 5′ end of the synthetic sequence, where ATA was the protective base and CATATG was the NdeI restriction site; GTTAACAGC was introduced at the 3′ end, where GTTAAC was the HpaI restriction site and AGC was the protective base. Then the synthetic anti-CD20 antibody kappa chain cDNA sequence was inserted into the light chain expression cassette of pIRES-105.
[0095] The cDNA sequence of the anti-CD20 antibody kappa chain and pIRES-105 plasmid DNA digested with NdeI / HpaI double enzyme digestion sy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com