Method for cheaply and extensively preparing nitrogen-based metal compound hydrogen storage material
A technology of hydrogen storage materials and compounds, applied in the direction of hydrogen production, etc., can solve the problems of difficult popularization and practical application, and achieve the effects of cost reduction, simple process, and strong safety and operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
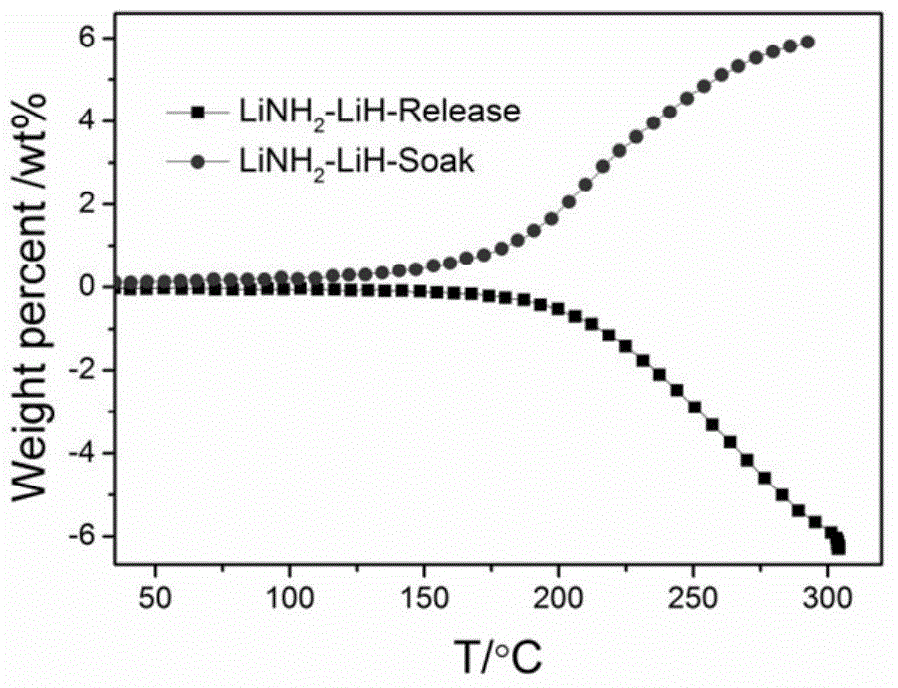
[0015] Example 1: Preparation of Li-N-H (LiNH 2 -LiH) system
[0016] (1) Weigh a certain amount of metallic lithium, such as 70g, in the glove box, then add it to the ball mill tank, and then inject 10-120atm ammonia gas or liquid ammonia.
[0017] (2) After adding lithium metal and ammonia gas as described in step (1), place the ball mill tank on a planetary ball mill at 100-200 rpm and mix for 1-20 hours, and at the same time make part of lithium metal react with ammonia gas to generate lithium amide.
[0018] (3) Take out the mixed sample in step (2) and place it in a pressure-resistant reactor for ammoniating. The ammoniating temperature is between 50-350℃; time is 1-50h; it can also be a long time (> 50h) Repeat step (2) until all metallic lithium is converted to lithium amide.
[0019] (4) As step (3), the lithium amide sample obtained after ammoniating is subjected to temperature program (fixed heating rate) deamination test (TPD-MS) to determine its deamination temperature, an...
Embodiment 2
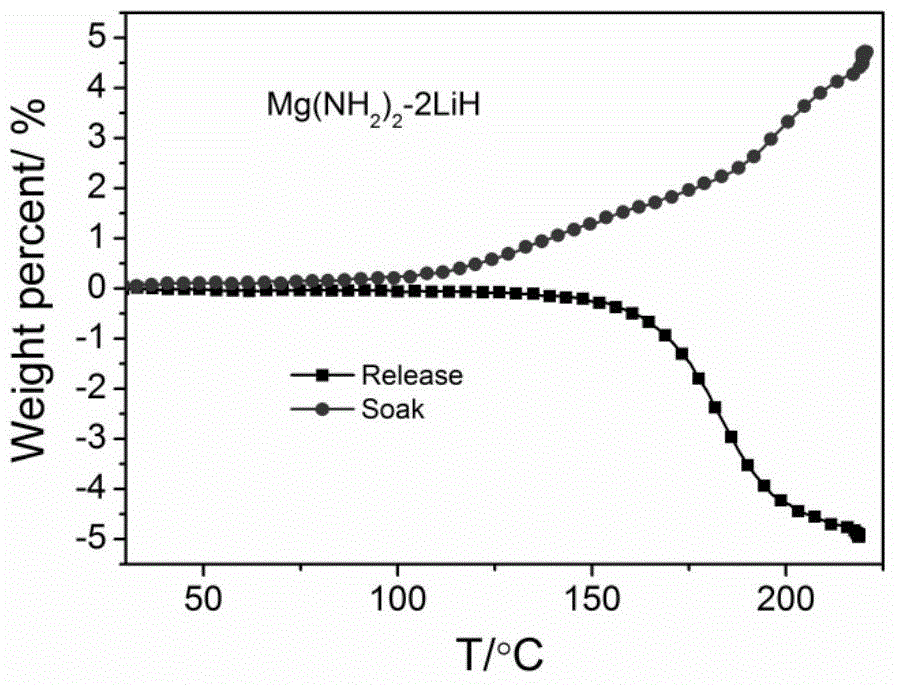
[0022] Example 2: Preparation of Li-Mg-N-H(Mg(NH 2 ) 2 -nLiH) system
[0023] (1) Weigh a certain amount of metallic magnesium and a corresponding amount of metallic lithium (such as Mg(NH 2 ) 2 -2LiH system, the molar ratio of Mg and Li is 1:2, such as a total of 38g), and then added to the ball mill tank, and then injected ammonia gas (> 5atm) or liquid ammonia.
[0024] (2) After adding magnesium metal, lithium metal and ammonia gas as described in step (1), place the ball mill on a planetary ball mill at 50-200 rpm and mix for more than 1 hour until uniform, and at the same time make part of metal lithium or magnesium react with ammonia gas This produces lithium amide or magnesium amide.
[0025] (3) Take out the mixed sample in step (2) and place it in a pressure-resistant reactor for ammoniating, and the ammoniating temperature is between 150 and 400°C;
[0026] (4) As in step (3), the sample obtained after ammoniating is subjected to temperature-programmed deamination test (TPD...
Embodiment 3
[0029] Example 3: Preparation of KH modified Li-Mg-N-H(Mg(NH 2 ) 2 -nLiH-yKH) system
[0030] (1) Weigh a certain amount of metallic magnesium and corresponding amount of metallic lithium and metallic potassium (such as Mg(NH) 2 ) 2 -1.9LiH-0.1KH system, the molar ratio of Mg to Li and K is 1:1.9:0.1, for example, the total weight is 41.2g), then add to the ball mill tank, and then inject ammonia gas (> 2atm) or liquid ammonia.
[0031] (2) After adding magnesium metal, lithium metal, potassium metal and ammonia as described in step (1), place the ball mill pot on a planetary ball mill at 50 to 200 rpm to mix> 1h, until the mixing is uniform, and at the same time part of the metallic lithium, metallic potassium or magnesium reacts with ammonia gas to generate lithium amide, potassium amide or magnesium amide.
[0032] (3) Take out the mixed sample in step (2) and place it in a pressure-resistant reactor for ammoniating, and the ammoniating temperature is between 150 and 400°C;
[0033]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com