Cation exchange chromatographic purification method anti-TNF alpha-type monoclonal antibody
A cation exchange and monoclonal antibody technology, which is applied in the preparation method of peptides, chemical instruments and methods, anti-animal/human immunoglobulin, etc., can solve the inconvenience of polymer removal, antibody denaturation and inactivation, high concentration of additives, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
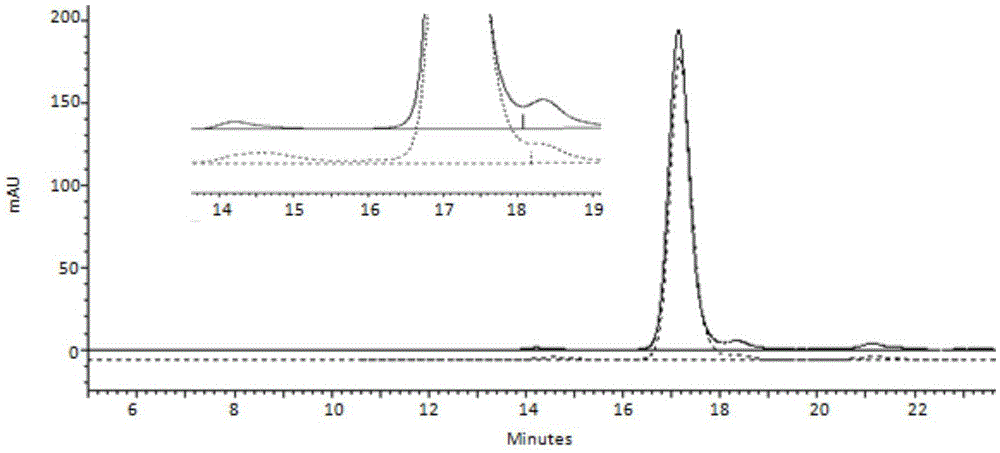
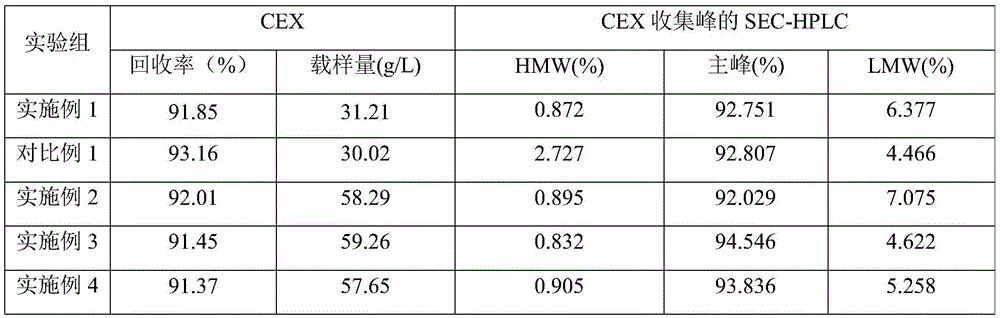
Examples
Embodiment 1
[0046] 1) Fermentation broth acquisition
[0047] CHO cells (Chinese hamster ovary cells) expressing fully human adalimumab were cultured and expressed adalimumab, and the expressed antibody was centrifuged at 4000-8000g for 10-15min on a SIGMA6K15 centrifuge to separate cells and For the fermentation broth, the supernatant was centrifuged for the second time, centrifuged at 6000-8000g for 10-15min to remove cell debris, and the supernatant was transferred to the next step.
[0048] Filtration: The supernatant after twice centrifugation is filtered through a 0.45μm filter membrane, and the filtered feed liquid can be directly loaded on the cationic column after adjustment.
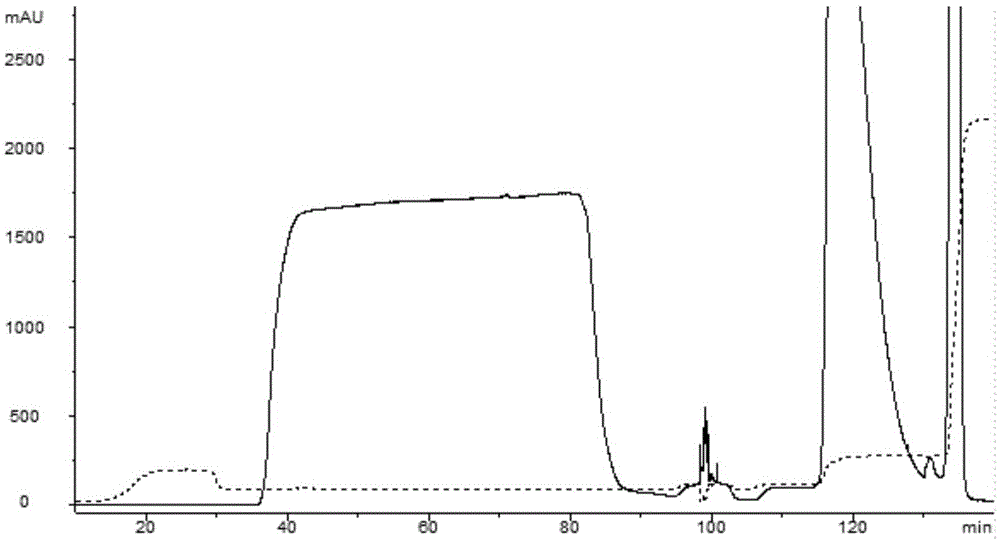
[0049] 2) Cation exchange column (CEX)
[0050] 1) Sample adjustment: Dilute the clarified feed solution by 1 time, adjust the pH to 6.0 with 0.5M citric acid, and the volume is about 210ml at this time;
[0051] 2) Equilibration: AKTA purifer, a chromatography system of GE Company, is used, and an XK16 / ...
Embodiment 2
[0062] 1) Fermentation broth acquisition
[0063] CHO cells (Chinese hamster ovary cells) expressing fully human adalimumab were cultured and expressed adalimumab, and the expressed antibody was centrifuged at 4000-8000g for 10-15min on a SIGMA6K15 centrifuge to separate cells and For the fermentation broth, the supernatant was centrifuged for the second time, centrifuged at 6000-8000g for 10-15min to remove cell debris, and the supernatant was transferred to the next step.
[0064]Filtration: The supernatant after twice centrifugation is filtered through a 0.45μm filter membrane, and the filtered feed liquid can be directly loaded on the cationic column after adjustment.
[0065] 2) Cation exchange column (CEX)
[0066] 1) Sample adjustment: Dilute the clarified feed solution by 1 time, adjust the pH to 6.0 with 0.5M citric acid, and the volume is about 400ml at this time;
[0067] 2) Equilibration: AKTA purifer, a chromatography system of GE Company, is used, and an XK16 / 2...
Embodiment 3
[0076] 1) Fermentation broth acquisition
[0077] CHO cells (Chinese hamster ovary cells) expressing fully human adalimumab were cultured and expressed adalimumab, and the expressed antibody was centrifuged at 4000-8000g for 10-15min on a SIGMA6K15 centrifuge to separate cells and For the fermentation broth, the supernatant was centrifuged for the second time, centrifuged at 6000-8000g for 10-15min to remove cell debris, and the supernatant was transferred to the next step.
[0078] Filtration: The supernatant after twice centrifugation is filtered through a 0.45μm filter membrane, and the filtered feed liquid can be directly loaded on the cationic column after adjustment.
[0079] 2) Cation exchange column (CEX)
[0080] 1) Sample adjustment: Dilute the clarified feed solution by 1 time, adjust the pH to 6.0 with 0.5M citric acid, and the volume is about 400ml at this time;
[0081] 2) Equilibration: AKTA purifer, a chromatography system of GE Company, is used, and an XK16 / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com