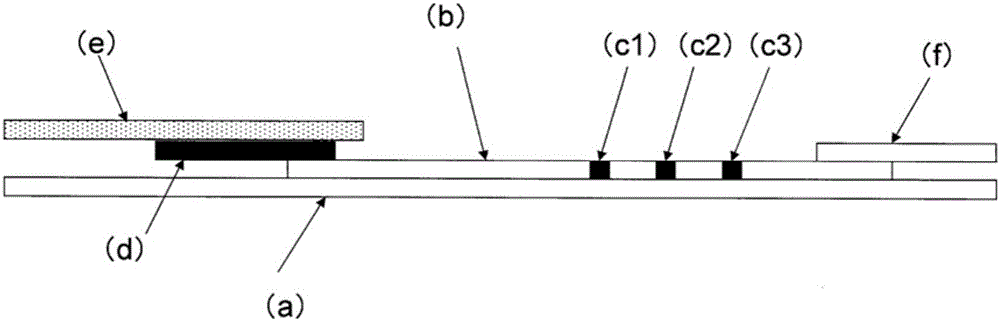
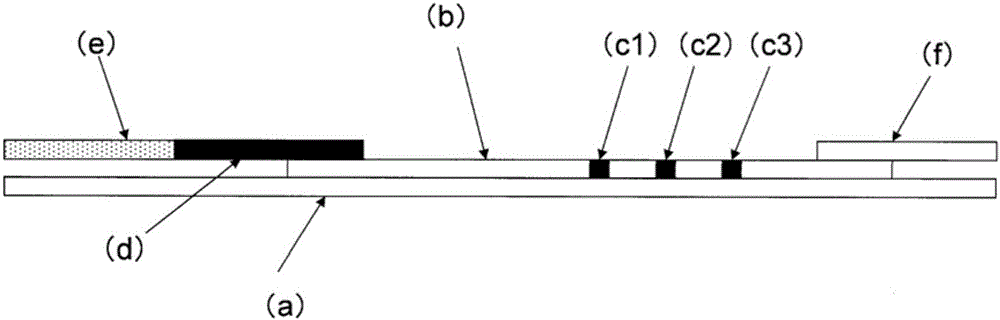
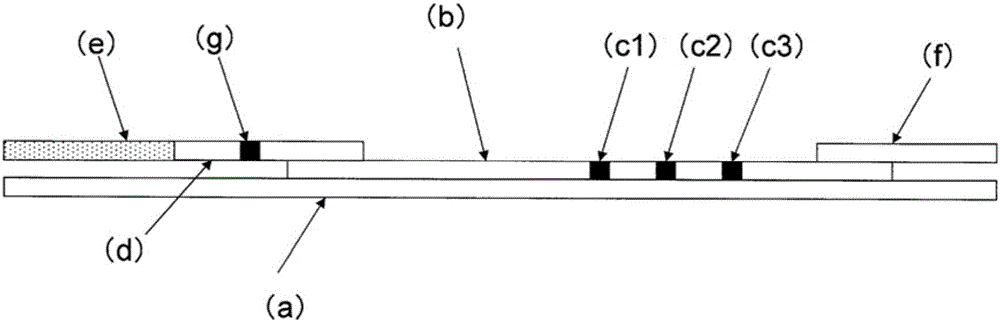
Immunochromatography-assisted detection method
An immunochromatographic detection, immunochromatographic technology, applied in immunoassays, measuring devices, analytical materials, etc., can solve the problem of unknown methods to eliminate this problem, low detection sensitivity of influenza B virus, unknown elimination of such bleaching phenomena, methods, etc., to achieve the effect of accurate measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Example 1 (bleaching evaluation test)
[0116] Bleaching was evaluated by using an immunochromatographic test strip (manufactured by Sekisui Medical Co., Ltd., Rapid Testa (registered trademark) ColorFLUStick, Lot. K1221221077P) stored unopened for two years at the temperature described in the package insert. Add glycerol or methanol to the sample-diluent (Tris buffer (pH 8.5)) attached to the test strip, respectively, to prepare sample dilutions containing 1%, 5% and 10% glycerol or 1%, Sample dilutions of 5% and 10% methanol. A set (n=3) of immunochromatographic test strips was immersed in each sample dilution to allow the sample dilution to run on the chromatograph for 10 minutes. Then, each test strip was visually inspected for the occurrence of bleaching. The results are shown in Table 1.
[0117] Similarly, the same test was performed submerging the test strips in sample dilutions containing 1%, 5% and 10% ethanol. The results are shown in Table 2.
[0118] "...
Embodiment 2
[0127] Embodiment 2 (accelerated test)
[0128] The immunochromatographic test strips obtained immediately after preparation and the immunochromatographic test strips stored at 60° C. for 10 days and 20 days were immersed in sample diluents containing 5% and 10% methanol to allow the solutions to be as described in Example 1. The situation was developed for 10 minutes and the occurrence of bleaching was checked visually. The results are shown in Table 3.
[0129] [table 3]
[0130]
[0131] d: number of days
[0132] (result)
[0133] Inhibition of the occurrence of bleaching and a reduction in the extent of bleaching was observed in all immunochromatographic test strips used immediately after preparation and after storage at 60°C for 10 and 20 days when sample diluents containing 5% and 10% methanol were used . In particular, a significant inhibition of the occurrence of bleaching was observed when using a sample diluent containing 10% methanol.
Embodiment 3
[0134] Embodiment 3 (comparison of tinting strength in A line and B line)
[0135] Compare the intensity of staining in "Line A" and "Line B" using immunochromatographic test strips. Prepare samples by mixing 330 μL of sample diluent containing 5% and 10% methanol with 50 μL of sample inactivated by diluting influenza A and influenza B virus strains at the dilution factors described in Tables 4 and 5 Viral antigens (respective types of influenza viruses are described in the table below). A set (n=2) of test strips was submerged in 135 [mu]L of the sample to which the respective inactivated viral antigen was added, allowing the sample dilutions to spread on the test strips for 10 minutes. Then, based on the color samples, the coloring intensity at the detection portion of each virus was obtained. The results for influenza A virus and influenza B virus are shown in Table 4 and Table 5, respectively.
[0136] [Table 4]
[0137]
[0138] Conv.: Conventional
[0139] Meth.:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com