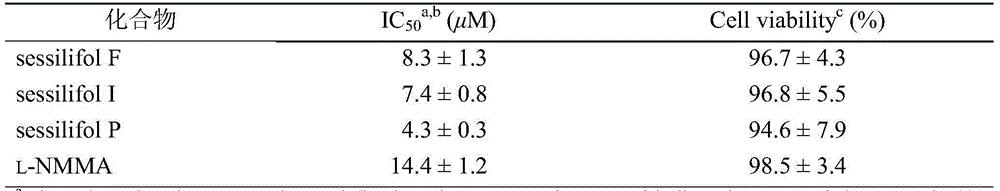Abietane type diterpene enantiomer compounds, and preparation method and application thereof
A compound, rosinane technology, applied in the field of medicine, can solve the problems of chemical composition and biological activity without any reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
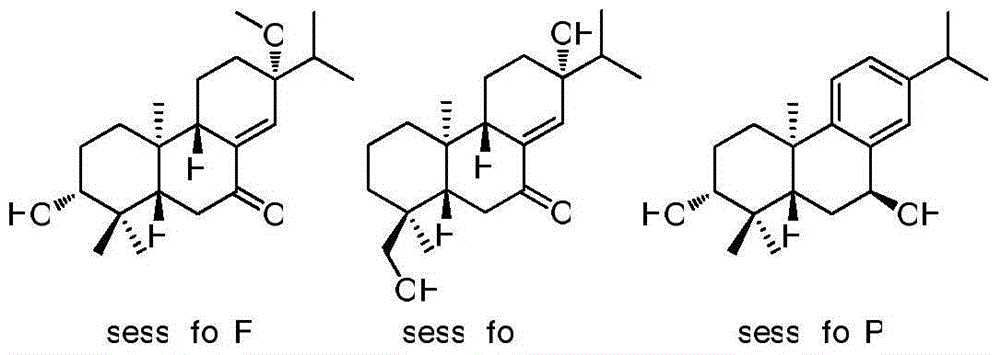
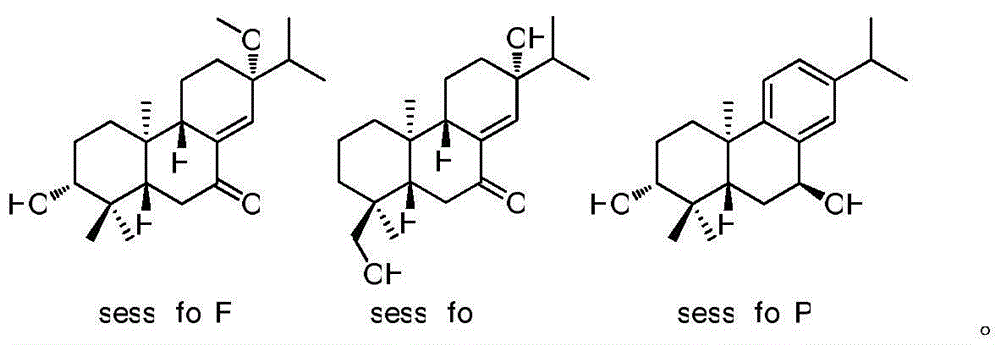
[0017] 6.8 kg of the whole plant of Sichuan Chrysanthemum orchids was soaked in 20L of pure methanol at room temperature for 24 hours, a total of 3 times. Combine 3 extracts afterwards, concentrate under reduced pressure, obtain 463 grams total extract. The obtained extract was dispersed with 2L of water, and then extracted with petroleum ether, ethyl acetate and n-butanol in sequence. 248 grams of all ethyl acetate fractions were preliminarily segmented with a large silica gel column (10×80cm; 100-200mesh), and the eluent was petroleum ether and acetone (from 9:1 to 0:1, v / v ), to obtain 6 components Fr.A-Fr.F. After Fr.A was separated by silica gel column for many times, it was finally purified by semi-preparative HPLC to obtain sessilifolF (1.8mg, t R =19.1min) and sessilifolI (1.7mg, t R = 22.8 min). Fr.D was further fractionated using MCI column (6.0×30cm), and the eluents were methanol and water (from 50% to 100%, v / v). The 70-80% methanol part was gradient eluted w...
Embodiment 2
[0022] Example 2: Anti-neuroinflammatory activity test
[0023] Mouse microglial BV-2 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM (Dulbecco'smodified Eagle's medium) medium (including 1800 mg / L NaHCO 3 , 10% (v / v) heat-inactivated fetal bovine serum (FBS), 100unit / mL penicillin G sodium salt and 100μg / mL streptomycin), 37°C, 5% CO 2 environment, in the incubator. The amount of NO produced was determined by Griess kit (Beyotime Biotechnology, China), that is, BV-2 cells were pretreated with different concentrations of test compounds for 4 h, and then added lipopolysaccharide (LPS, 1 μg / ml, Sigma-Aldrich) and incubated for 24 h (blank group without LPS). Aspirate the culture supernatant, mix it with an equal amount of Griess reagent, let it stand at room temperature in the dark for 10 minutes, measure the OD value of each group at a wavelength of 540nm with a microplate reader (M200, TECAN, AustriaGmbH, Austria), and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com