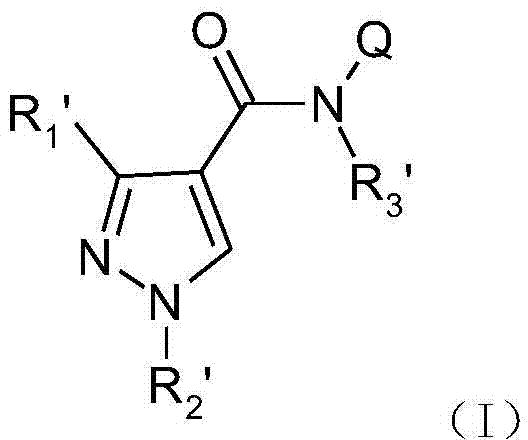
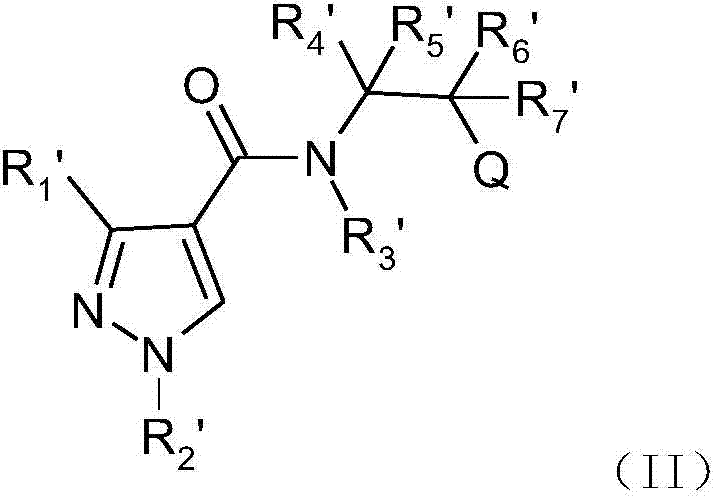
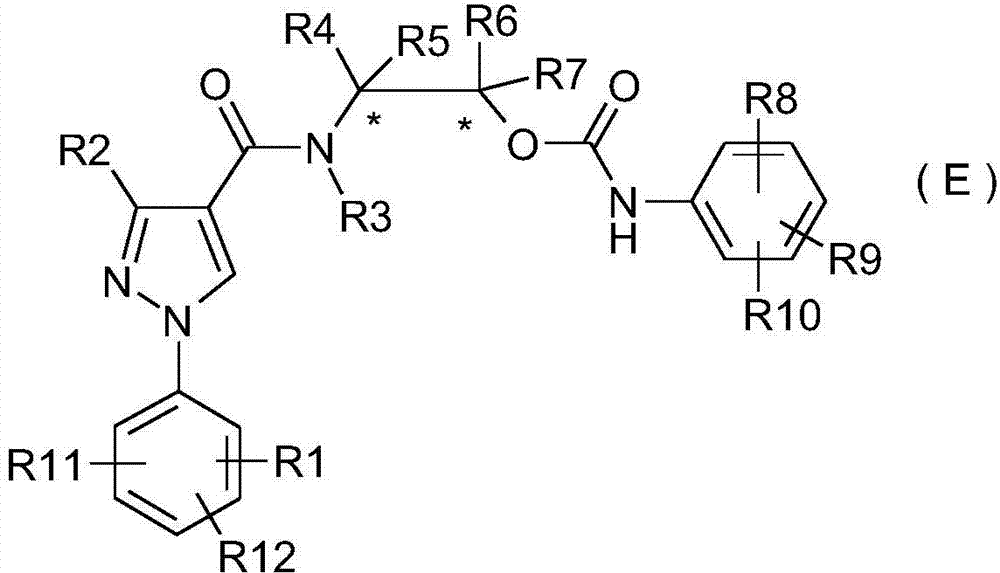
A class of phenylpyrazole amide derivatives, its preparation method and application
A technology of phenylpyrazole amide and alkyl, which is applied in the field of phenylpyrazole amide derivatives, and can solve problems such as not being disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: the preparation of intermediate (C-1)
[0084] Dry the reaction device, put 10 mmoles of 2-amino-1-propanol (B), 12 mmoles of triethylamine, and 100 ml of dichloromethane into a single-necked flask, and slowly drop 10 mmoles of 3-difluoromethane in an ice bath Dichloromethane solution of 1-(2,6-2 chloro-4-trifluoromethylphenyl)-1H-pyrazole-4-carbonyl chloride (A) in 30ml of dichloromethane, stirred at room temperature for 1 hour after dropping, and placed After 3 hours, separated by TLC (developing solvent: ethyl acetate), a white solid (C-1)3-(difluoromethyl)-N-(1-hydroxypropan-2-yl)-1-phenyl-1H-pyrazole was obtained -4-carboxamide.
[0085] The above (B) can also be replaced with 2-amino-1-propanol (L type), 2-amino-2-methyl-1-propanol, aminoethanol, etc. to obtain the corresponding intermediate (C-1).
Embodiment 2
[0086] Embodiment 2: the preparation of target compound (E-2)
[0087] Weigh 10 millimoles of the intermediate (C-1) prepared in Example 1 in a single-necked flask, use 20 ml of tetrahydrofuran as a solvent, and drop into 10 millimoles of 2-methyl-3-chloro-phenylisocyanate (D), Stir overnight at room temperature, concentrate, and separate by TLC (developing solvent is a mixture of V (ethyl acetate): V (petroleum ether) = 4:1) to obtain the target product (E-2), whose NMR data are as follows:
[0088] NMR 1 H NMR (600MHz, CDCl 3): δ8.04(s,1H),7.72(s,2H),7.51(t,J=54Hz,1H),7.19(d,J=7.9Hz,1H),7.10(t,J=8.1Hz, 1H),6.94(s,1H),6.82(s,1H),4.52-4.47(m,1H),4.38(dd,J=11.3,7.9Hz,1H),4.20(dd,J=11.6,3.4Hz ,1H),2.26(s,3H),1.31(d,J=6.5Hz,3H).
[0089] All compounds E-1 to E-107 can be synthesized by the same method.
[0090] 2. Preparation
Embodiment 3
[0091] Embodiment 3: Wettable powder formulation
[0092] 15% compound (E-a or E-b), 5% lignosulfonate (M q ), 1% lauryl polyoxyethylene ether (JFC), 35% diatomaceous earth and 44% light calcium carbonate are evenly mixed and pulverized to obtain a wettable powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com