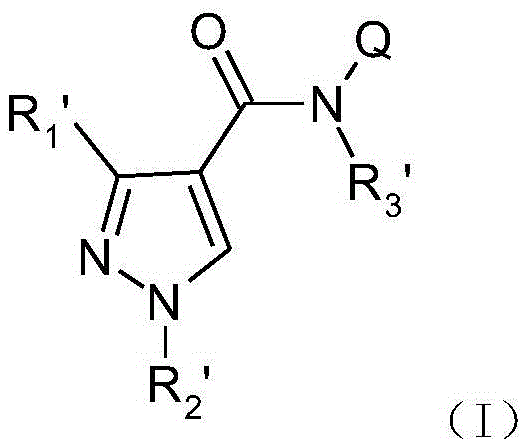
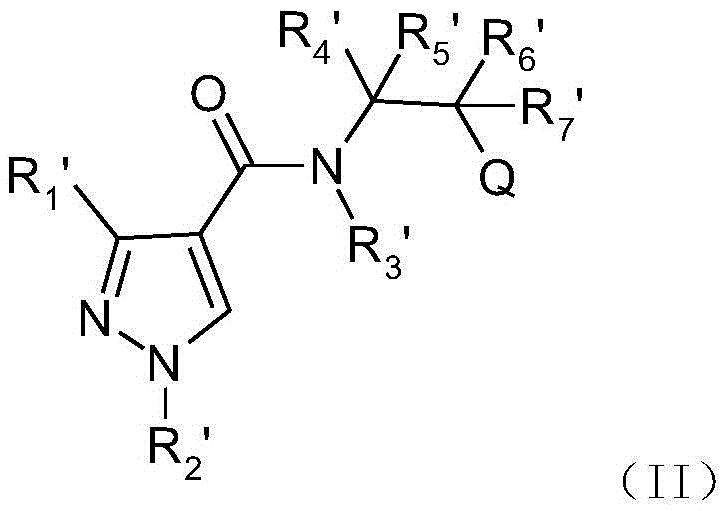
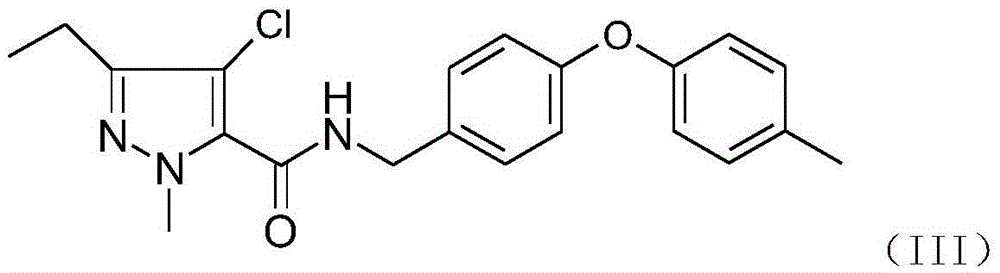
Pyrazole amide derivatives, preparation method and application thereof
A pyrazole amide and formamide-based technology, applied in the field of pyrazole amide derivatives, can solve the problems of undisclosed compounds, undisclosed agrochemical nematicide activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 intermediate (C-1)
[0059] Dry the reaction device, put 3.75 grams of 2-amino-1-propanol (B-1), 6.3 grams of triethylamine, and 50 ml of dichloromethane into a single-necked flask, slowly drop 11.20 grams of 3-ethyl-1- Methyl-4-chloro-1H-pyrazole-5-formyl chloride (A-1) in 50ml of dichloromethane solution, stirred at room temperature for 1 hour after dripping, left for 3 hours, separated by TLC after precipitation (developing solvent: acetic acid ethyl ester), to obtain 10.9 g of white solid (C-1). The obtained white solid (C-1) was 3-ethyl-N-(1-hydroxypropyl-2-yl)-1-methyl-1H-pyrazole-5-carboxamide.
[0060] The above (B) can also be replaced with 2-amino-1-propanol (swirl), 2-amino-2-methyl-1-propanol, aminoethanol, etc. to obtain the corresponding intermediate (C).
Embodiment 2
[0061] The preparation of embodiment 2 target compound (E-1)
[0062] Weigh 10mmol of the intermediate (C-1) prepared in Example 1 and 10mmol of 4-heptafluoroisopropylphenylisocyanate (D-1) in a single-necked flask, use 20ml of tetrahydrofuran as a solvent, stir overnight at room temperature, concentrate, and TLC Separation (the developing solvent is a mixture of V (ethyl acetate): V (petroleum ether) = 4:1) to obtain 2.50 g of the target product (E-1). The NMR data of the target product (E-1) are as follows:
[0063] NMR: 1 H-NMR (ppm):
[0064] δ1.22~1.27(t,3H),1.33~1.35(d,3H),2.60~2.64(q,2H),4.10(s,3H),4.26~4.29(m,2H),4.50~4.52(m ,1H), 6.77~6.78(d,1H), 7.01(s,1H), 7.52~7.55(m,4H).
[0065] All compounds from E-1 to E-95 can be synthesized using the same method.
[0066] 2. Preparation
Embodiment 3
[0067] Embodiment 3 wettable powder formula
[0068] In terms of mass percent, 15% of the compound (compounds described in Table 1 and Table 2), 5% of lignosulfonate (M q ), 1% lauryl polyoxyethylene ether (JFC), 40% diatomaceous earth and 44% light calcium carbonate are evenly mixed and pulverized to obtain a wettable powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com