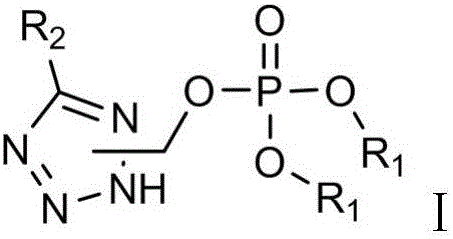
Tetrazole phosphate compound with nematocidal activity and synthesizing method
A technology of tetrazolium phosphate and azole phosphate, applied in the field of organic compound synthesis, can solve the problems of less cross-resistance, short residual effect period, easy to be degraded and the like, achieve good nematicidal activity, low cost, and easy realization of reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
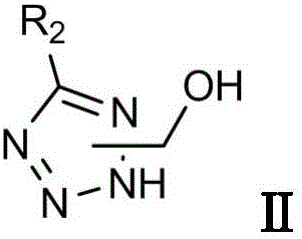
[0037] (1) Preparation of intermediate 1-hydroxymethyl-5-benzyltetrazole Ⅱ-1
[0038] To a dry reaction flask with a thermometer, add 2 mL of N,N-dimethylformamide and 0.30 g (3 mmol) of 1-hydroxymethyl tetrazole, 0.40 g (3.17 mmol) of benzyl chloride and 0.62 g (4.50 mmol) of Potassium carbonate water (by molar ratio, hydroxymethyl tetrazole:halogenated compound:alkali=1:1.06:1.5), slowly heated to 100°C under stirring to react overnight. The reaction was cooled to room temperature and poured into 8 mL of water. Transfer to a separatory funnel, extract with ethyl acetate, collect the organic phase, wash with water, wash with brine, dry, and filter. The filtrate was concentrated to dryness under reduced pressure to obtain a crude product, which was purified on a silica gel column with ethyl acetate: petroleum ether = 1:5 (v / v) to obtain the intermediate 1-hydroxymethyl-5-benzyltetrazole II-1, which was white Solid, yield 43%, structural formula is as follows:
[0039]
...
Embodiment 2
[0048] (1) Preparation of intermediate 2-hydroxymethyl-5-benzyltetrazole II-2
[0049]To a dry reaction flask with a thermometer, add 2 mL of N,N-dimethylformamide and 0.30 g (3 mmol) of 2-hydroxymethyl tetrazole, 0.40 g (3.17 mmol) of benzyl chloride and 0.68 g (4.95 mmol) of Potassium carbonate water (in molar ratio, hydroxymethyl tetrazole:halogenated compound:alkali=1:1.06:1.65), slowly heated to 100°C under stirring to react overnight. The reaction was cooled to room temperature and poured into 8 mL of water. Transfer to a separatory funnel, extract with ethyl acetate, collect the organic phase, wash with water, wash with brine, dry, and filter. The filtrate was concentrated to dryness under reduced pressure to obtain a crude product, which was purified on a silica gel column with ethyl acetate: petroleum ether = 1:5 (v / v) to obtain the intermediate 2-hydroxymethyl-5-benzyltetrazole II-2, which was white Solid, yield 44.5%, structural formula is as follows:
[0050] ...
Embodiment 3
[0059] (1) Preparation of intermediate 2-hydroxymethyl-5-p-chlorobenzyltetrazole Ⅱ-3
[0060] In a dry reaction flask with a thermometer, add 2mL N, N-dimethylformamide and 0.30g (3mmol) 2-hydroxymethyltetrazole, 0.51g (3.17mmol) p-chlorobenzyl chloride and 0.51g (5.0mmol) ) triethylamine (in molar ratio, hydroxymethyltetrazole:halogenated compound:alkali=1:1.06:1.67), slowly heated to 100°C under stirring and reacted overnight. The reaction was cooled to room temperature and poured into 8 mL of water. Transfer to a separatory funnel, extract with ethyl acetate, collect the organic phase, wash with water, wash with brine, dry, and filter. The filtrate was concentrated to dryness under reduced pressure to obtain a crude product, which was purified on a silica gel column with ethyl acetate: petroleum ether = 1:5 (v / v) to obtain the intermediate 2-hydroxymethyl-5-p-chlorobenzyltetrazole II-3, It is a white solid with a yield of 40%. The structural formula is as follows:
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com