Electronic transmission material and its preparation method and use
A technology of electron transport materials and electron transport layers, which is applied in the direction of circuits, electrical components, electric solid devices, etc., can solve the problems of rare electron transport materials, achieve high-efficiency electroluminescence performance, high fluorescence quantum efficiency, and avoid compactness. cumulative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
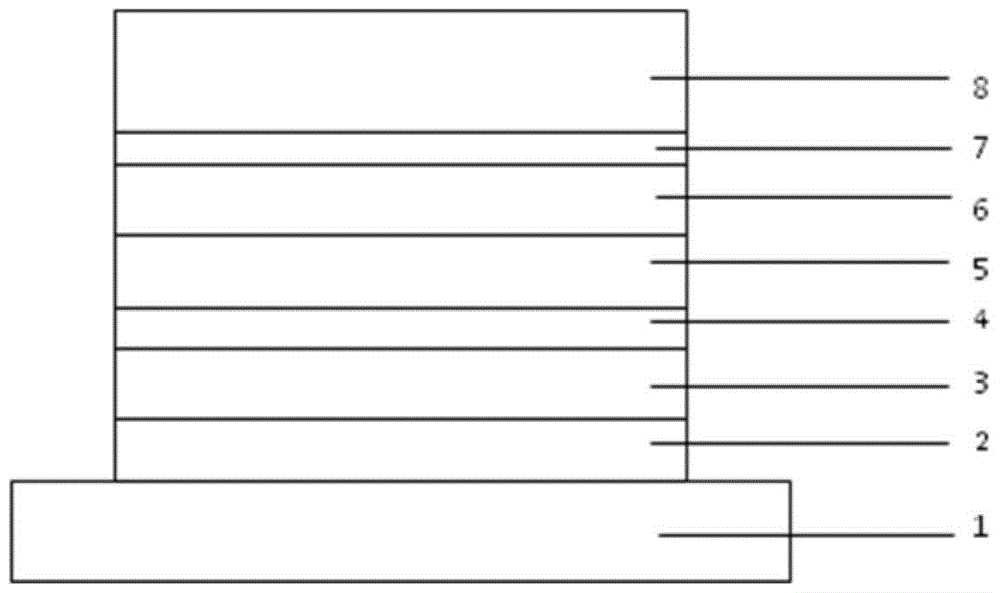
Image
Examples
Embodiment 110
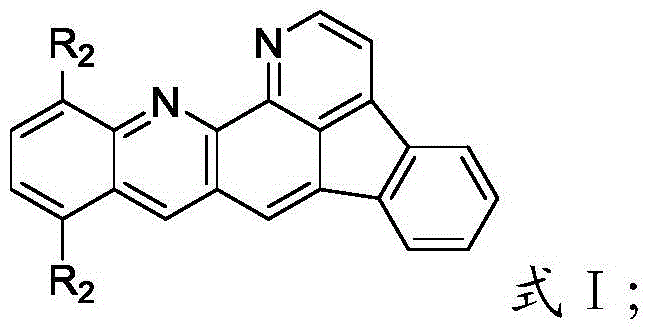
[0047] Synthesis of embodiment 110,13-bis (2-naphthyl) benzindole [1,2,3-gh] [1,10] phenanthroline (compound 1):
[0048]
[0049] Preparation of compound 1:
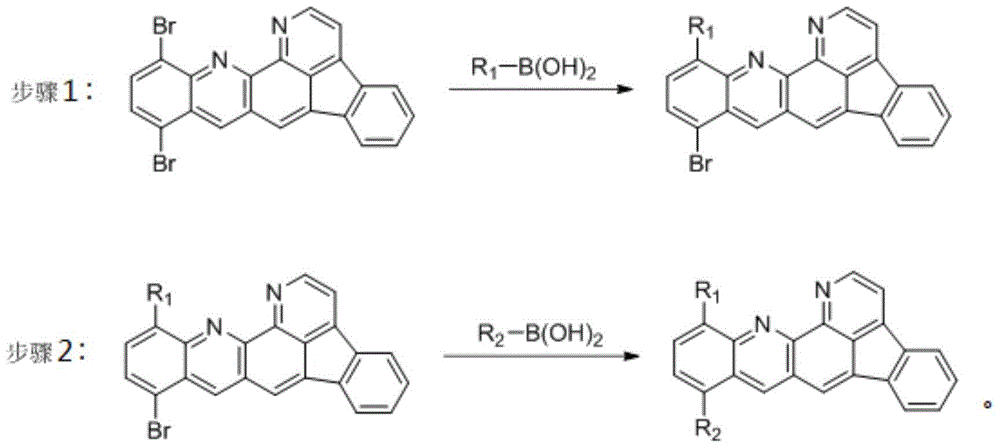
[0050] Take 10,13-dibromobenzindole [1,2,3-gh] [1,10] phenanthroline (4.6g, 10.0mmol), 2-naphthylboronic acid (3.8g, 22.0mmol), potassium carbonate (4.2g, 30.0mmol) and 10g of water were dissolved with 60mL of toluene and 30mL of ethanol, and stirred under nitrogen gas for 1 hour to remove the oxygen in the reaction flask. Then add Pd(PPh3) 40.230g (2.0mmol), reflux under vigorous stirring, and the reaction process is tracked and detected by TLC. After the reaction was completed, the aqueous phase was extracted with 200 mL of ethyl acetate, and the organic phase was desolvated under reduced pressure until there was no fraction. The residue was purified by column chromatography with pure toluene, and then recrystallized by toluene ethanol to obtain compound 1 (3.9 g, 70.14%) . The crude product was further sublimat...
Embodiment 213
[0051] Example 21 3-(2-naphthyl)-10-(4-(2-naphthyl)phenyl)benzindole[1,2,3-gh][1,10]phenanthroline (compound 2) Synthesis:
[0052]
[0053] Preparation of Compound A:
[0054] Take 10,13-dibromobenzindole [1,2,3-gh] [1,10] phenanthroline (4.6g, 10.0mmol), 2-naphthylboronic acid (1.4g, 8.0mmol), potassium carbonate (2.1g, 15.0mmol) and 10g of water were dissolved with 60mL of toluene and 30mL of ethanol, and stirred under nitrogen gas for 1 hour to remove the oxygen in the reaction flask. Then add Pd(PPh3) 40.115g (1.0mmol), reflux under vigorous stirring, and the reaction process is tracked and detected by TLC. After the reaction was completed, the aqueous phase was extracted with 200 mL of ethyl acetate, and the organic phase was desolvated under reduced pressure until there was no fraction. The residue was purified by column chromatography with pure toluene, and recrystallized by toluene ethanol to obtain compound A (2.4 g, 46.80%) .
[0055] Preparation of compound ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com